Abstract
Purpose
The explosive progression of residual hepatocellular carcinoma (HCC) following incomplete thermal ablation is challenging, and the underlying mechanisms require further exploration. We investigated the mechanism by which Forkhead box P4 (FOXP4) promotes the malignant transformation of residual HCC cells through N-deacetylase and N-sulfotransferase 2 (NDST2) after incomplete thermal ablation.Methods
The clinical significance of FOXP4 and NDST2 in HCC was evaluated using big data analysis. FOXP4 expression was detected in clinical samples of HCC. The gene expression levels in an in vitro heat-stressed HCC cell model were determined using quantitative real-time PCR (RT-qPCR) and Western blotting. The effects of the genes on heat-stressed HCC cells were investigated using Cell Counting Kit-8 (CCK-8), scratch, Transwell migration, and invasion assays. Additionally, the regulatory relationship between FOXP4 and NDST2 was validated using the Cleavage Under Targets and Tagmentation (CUT&Tag) experiments and phenotypic assays.Results
High FOXP4 expression was correlated with liver cancer occurrence and development. In the heat-stressed HCC cell model, downregulating FOXP4 inhibited cancer cell progression. Besides, there was a positive association between FOXP4 and NDST2 in liver cancer. Suppressing FOXP4 reduced NDST2 expression in the heat-stressed HCC cells. Furthermore, reducing NDST2 expression weakened the biological behavior of heat-stressed HCC cells.Conclusion
FOXP4 and NDST2 are crucial in the incomplete thermal ablation of residual cancer. FOXP4 might regulate the biological progression of residual HCC after incomplete thermal ablation through NDST2.Free full text

Incomplete Thermal Ablation-Induced FOXP4-Mediated Promotion of Malignant Progression in Liver Cancer via NDST2
Abstract
Purpose
The explosive progression of residual hepatocellular carcinoma (HCC) following incomplete thermal ablation is challenging, and the underlying mechanisms require further exploration. We investigated the mechanism by which Forkhead box P4 (FOXP4) promotes the malignant transformation of residual HCC cells through N-deacetylase and N-sulfotransferase 2 (NDST2) after incomplete thermal ablation.
Methods
The clinical significance of FOXP4 and NDST2 in HCC was evaluated using big data analysis. FOXP4 expression was detected in clinical samples of HCC. The gene expression levels in an in vitro heat-stressed HCC cell model were determined using quantitative real-time PCR (RT-qPCR) and Western blotting. The effects of the genes on heat-stressed HCC cells were investigated using Cell Counting Kit-8 (CCK-8), scratch, Transwell migration, and invasion assays. Additionally, the regulatory relationship between FOXP4 and NDST2 was validated using the Cleavage Under Targets and Tagmentation (CUT&Tag) experiments and phenotypic assays.
Results
High FOXP4 expression was correlated with liver cancer occurrence and development. In the heat-stressed HCC cell model, downregulating FOXP4 inhibited cancer cell progression. Besides, there was a positive association between FOXP4 and NDST2 in liver cancer. Suppressing FOXP4 reduced NDST2 expression in the heat-stressed HCC cells. Furthermore, reducing NDST2 expression weakened the biological behavior of heat-stressed HCC cells.
Conclusion
FOXP4 and NDST2 are crucial in the incomplete thermal ablation of residual cancer. FOXP4 might regulate the biological progression of residual HCC after incomplete thermal ablation through NDST2.
Introduction
Hepatocellular carcinoma (HCC) is a prevalent malignancy globally, with diverse causes that include viral infections, long-term alcohol consumption, genetic factors, and environmental factors.1,2 HCC symptoms typically include pain in the liver area, loss of appetite, fatigue, weight loss, and fever. Surgical resection is one of the main treatment methods for HCC. However, it is only effective if the tumor is small and localized to a specific liver region. For larger tumors or cases where cancer has spread to multiple regions of the liver, combination therapies, including chemotherapy, radiation therapy, and immunotherapy, can be employed to alleviate symptoms and prolong the life of patients.3,4
The continuous development of medical technology has resulted in thermal ablation therapy gradually becoming a new type of local treatment for HCC.5 Thermal ablation therapy includes radiofrequency, microwave, and laser ablation. The principle of treatment is to convert energy into heat, causing HCC tissue necrosis and achieving the desired therapeutic effect.6 The advantages of thermal ablation therapy are minimal invasiveness, quick postoperative recovery, and good treatment outcomes. Thermal ablation therapy can also improve patient survival rates and liver function while reducing recurrence rates.7
In HCC treatment, thermal ablation therapy can be used as an adjuvant treatment before, during, and after surgery. The specific application depends on the size, location, and number of liver cancers. For patients with small liver tumors, the complete necrosis rate of thermal ablation therapy can exceed 90% while minimizing trauma and enabling fast postoperative recovery. For patients with massive HCC, the combined thermal ablation and surgical resection treatments yield significantly better outcomes than surgical resection alone while reducing recurrence rates.8 Moreover, combining thermal ablation and surgical resection can achieve favorable treatment outcomes for patients with malignant liver tumors. However, incomplete ablation may occasionally occur in thermal ablation therapy for HCC due to the pathological characteristics of the tumor, its specific location, and technical challenges.9 Residual tumor tissue resulting from incomplete ablation can lead to tumor persistence and recurrence.10 Inflammation, epithelial-mesenchymal transition (EMT), tumor stem cells, angiogenesis, autophagy, and the tumor microenvironment might be associated with the residual cancer progression.11 The influences and mechanisms behind this phenomenon remain under active research and exploration.
Forkhead box P4 (FOXP4) is classified under the P subfamily of the Fox transcription factor family and functions as a transcription factor. FOXP4 contains an N-terminal C2HC-type zinc finger structure and a C-terminal forkhead domain and can bind DNA.12 A previous study has suggested a link between the FOXP4 gene and liver cancer onset and progression.13 Various HCC studies identified FOXP4 as an oncogenic transcription factor that can enhance HCC progression and metastasis. FOXP4 enhanced HCC cell growth and invasive capabilities, and elevated FOXP4 expression was noted in patients with HCC, suggesting a link between FOXP4 and HCC exacerbation.14 Additionally, FOXP4 can interact with other transcription factors and signaling pathways, further influencing HCC progression. For example, FOXP4 can be targeted by microRNA (miR)-4651, regulating HCC cell growth and apoptosis.15 The long non-coding RNA (lncRNA) LOC 105372579 interacts with miR-4316 to promote FOXP4 expression, regulating HCC proliferation and EMT.16 Nevertheless, no report exists on the role of FOXP4 in influencing the accelerated progression of heat-stressed HCC and its potential downstream regulatory factors.
N-deacetylase and N-sulfotransferase 2 (NDST2) is an N-deacetylase/N-sulfotransferase that primarily participates in sulfation and is crucial in the N-deacetylation of heparan sulfate (HS) glycosaminoglycans and N-sulfation of glucosamine.17,18 HS is a glycosaminoglycan extensively present on the cell surface and has diverse biological functions, including anticoagulation, anti-inflammation, and anti-tumor effects. Several studies established that HS can influence HCC progression through multiple mechanisms. HS forms HS proteoglycans by connecting with core proteins in organisms, which can resist tumor invasion by preventing tumor cell infiltration and cell-cell adhesion.19 Other studies noted that glypican-3, an HS proteoglycan family member, is a potential prognostic factor and immune target for HCC used for treatment and as an early detection marker for HCC.20,21 Previously, we investigated the significance of NDST2 in HCC and its implications on the accelerated progression of residual HCC following incomplete thermal ablation.22 The study revealed a connection between elevated NDST2 expression and the prognosis of heat-stressed HCC cells. Furthermore, downregulating NDST2 expression effectively hindered the rapid progression of heat-stressed HCC cells. Research on NDST2 in HCC and incomplete thermal ablation HCC is limited. Further studies are needed to reveal the targeted regulatory relationship of NDST2 in incomplete thermal ablation HCC and its specific role and mechanism.
No studies have been reported on the interaction between FOXP4 and NDST2 in residual HCC rapid progression following incomplete thermal ablation. However, our previous studies indicated that FOXP4 and NDST2 are significantly expressed in HCC and are closely associated with its occurrence and development. Extensive public data analysis revealed a potential association between FOXP4 and NDST2, indicating a potential regulatory relationship between these genes that may jointly contribute to the aggressive progression of residual HCC after incomplete thermal ablation. This study aimed to investigate the mechanisms and potential associations between FOXP4 and NDST2 in residual HCC after incomplete thermal ablation, thereby enhancing the understanding and practical guidance for HCC treatment and prognosis with thermal ablation and contributing to the advancement and development of HCC treatment.
Materials and Methods
Clinical Sample Collection
A comprehensive collection of 25 pairs of HCC and adjacent non-cancerous tissues was assembled from the First Affiliated Hospital of Guangxi Medical University between 2020 and 2022. The collected clinical samples were used quantitative reverse transcription polymerase chain reaction (RT-qPCR) experiments to detect FOXP4 expression. Ethical approval for this study (NO.2019-KY-(084)) was provided by the Ethical Committee of The First Affiliated Hospital of Guangxi Medical University. All research was conducted in accordance with the Declarations of Helsinki and Istanbul. All patients provided informed consent before undergoing surgery. The samples were stored at −80 °C until further use. Table S1 outlines the details of the clinical samples.
Big Data Analysis
The differential expression of relevant genes between the normal liver tissue and liver cancer tissue in the Cancer Genome Atlas (TCGA) database was analyzed using the Gene Expression Profiling Interactive Analysis (GEPIA) database. The overall survival (OS) of patients with HCC associated with these genes was evaluated using Kaplan-Meier analysis. The correlation between FOXP4 and NDST2 expression in the liver tissue and liver cancer was assessed using Spearman’s and Pearson’s correlation analysis.
Cell Cultivation
We procured human liver cancer cell lines (MHCC97-H and MHCC97-L) from Cellcook (Guangzhou, China). The cells were cultured in Dulbecco’s modified Eagle’s medium, to which 10% fetal bovine serum and 1% penicillin-streptomycin were added. The cells were incubated at 37 °C with 5% CO2 in a controlled environment.
Small Interfering RNA (siRNA) Design and Cell Transfection
Sangon Biotech (Shanghai) designed and synthesized specific siRNAs targeting the FOXP4 and NDST2 genes. Table S2 presents the siRNA synthesis report. The siRNAs were transfected into the liver cancer cells using the RNATransMate transfection reagent. The control was the empty transfection vector. Transfection efficiency was evaluated 48h post-transfection using quantitative real-time PCR (RT-qPCR) and Western blotting.
In vitro Simulation of Incomplete Thermal Ablation
The human liver cancer cells were plated in a six-well plate and cultured until 70–80% confluency. The six-well plate was placed in a water bath with carefully controlled temperature and duration to simulate incomplete cellular thermal ablation in vitro. The water bath temperature was set at 47 °C, and each treatment cycle lasted for 10 min. After the water bath cycle, the cells were re-cultured in the incubator until they regained 70–80% confluency before the next cycle began. After completing all heat treatment cycles, the cells were incubated for 48 h before the subsequent experiments. The procedures followed those reported in a previous study.23
RT-qPCR
Total RNA was extracted from the liver cancer cells and reverse-transcribed into complementary DNA. The target RNA expression was detected using the QuantStudio™ Real-Time PCR System. Table S3 lists the primer sequences used for the PCR.
Western Blotting
Changes in the FOXP4 and NDST2 protein levels were detected using Western blotting. A cell lysis buffer was prepared by combining a protease inhibitor mixture with a mammalian protein extraction reagent using a 1:99 ratio. The cells were lysed on ice using this buffer. The lysate mixture was collected and centrifuged at 14,000 rpm and 4 °C. The supernatant was transferred to a new 1.5 mL centrifuge tube. Gel preparation, protein sample loading, SDS-PAGE electrophoresis, and membrane transfer were performed to immobilize the proteins on the polyvinylidene fluoride membrane. The membrane was blocked for 1 h with 5% skimmed milk and incubated with the primary antibody at 4 °C overnight. The secondary antibody was incubated for 1 h at room temperature after washing the membrane thrice with PBST. The membrane was immersed in a freshly prepared chemiluminescent substrate for development, and images were captured using the ChemiDoc™ Imaging System. The primary antibodies used were anti-NDST2 (Bio-Techne) and anti-FOXP4 (Abcam), while the secondary antibody was horseradish peroxidase (HRP)-conjugated Affinipure goat anti-rabbit IgG(H+L) (Proteintech).
Cell Counting Kit-8 Assay (CCK-8)
Cells were seeded in a 96-well plate at a density of 3000 cells per well in 100 µL of the complete medium. At 6, 24, 48, 72, 96, and 120 h after seeding, the CCK-8 solution (10 µL per well) was added, and the plate was incubated in a cell culture incubator for 2 h, protected from light. The Varioskan Lux Multifunctional Enzyme Labeler was used to measure the absorbance at 450 nm.24
Transwell Migration Assay
The cells were collected and resuspended in a serum-free medium, and their concentration was measured. The cell suspension was diluted to 30,000 cells/200 µL. The upper chamber of a Transwell insert (Corning, 8 µm pore size) was filled with 200 µL of cell suspension in serum-free medium, while the lower chamber of the culture plate was filled with 800 µL of medium containing 10% serum. The cells were incubated in a cell culture incubator for 3 days before being fixed with 4% paraformaldehyde and stained with crystal violet. The crystal violet inside the insert was cleaned using a cotton swab. Fluorescence-inverted microscope was used to observe and photograph the cells. Image J software was used to count the cells.25
Transwell Invasion Assay
The upper chamber of a Transwell insert (Corning, 8 µm pore size) was pre-coated with 100 µL of a 1:10 diluted Matrigel and incubated at 37 °C for 1 h in a cell culture incubator. The residual non-invasive Matrigel in the upper chamber was removed, and the subsequent steps were identical to those of the Transwell migration assay.
Scratch Assay
Before seeding the cells in a six-well plate, a line was drawn on the back of the plate using a marker pen. When the cell density reached 80%-90%, a 200 µL yellow pipette tip was used to make a vertical scratch across the marked line. The cells suspended in the upper chamber were washed using PBS and cultured in a medium supplemented with 2% serum. Photographs were taken at 0, 24, and 48 h. Image J software was used to calculate the cell migration distance.26
Immunohistochemistry
The tissue microarray (HLivH160CS02) was obtained from Shanghai Outdo Biotech Co., Ltd (Shanghai, China). It comprised 80 pairs of HCC tissues and their corresponding adjacent tissues. The immunohistochemistry was primarily conducted according to the instructions of the PV-9000 Universal Two-Step Detection Kit (Mouse/Rabbit Enhanced Polymer Detection System). Immunohistochemistry was performed using a FOXP4 antibody (Abcam) and a broad-spectrum secondary antibody (Proteintech). Image J software was used to analyze and process the stained immunohistochemical images. One pair of sample with missing cancerous tissue was excluded, leaving 79 pairs of samples for analysis. Table S4 presents the information on the tissue microarray samples.
CUT&Tag Assay
The FOXP4 protein-DNA interaction was studied using the Cleavage Under Targets and Tagmentation (CUT&Tag) assay. The highly active Tn5 transposase was fused with protein A to form the pA-Tn5 transposase complex using molecular biology techniques. Guided by an antibody, this complex can target and cleave DNA sequences near the target protein. The experimental data underwent quality control, data characteristic analysis, and visualization of gene peaks.
Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics 23. The data were visualized using GraphPad Prism 8. Statistical differences between the two groups of data were analyzed using the paired t-test, with P < 0.05 considered statistically significant (*P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001). All experiments were repeated at least three times. The data are presented as the mean ± standard deviation (SD).
Results
Expression and Clinical Significance of FOXP4 in HCC
FOXP4 expression in HCC tumors and adjacent non-cancerous tissues was evaluated using RT-qPCR and immunohistochemistry assays. The RT-qPCR of 25 pairs of clinical samples revealed that, compared to the matched normal tissues, FOXP4 expression was significantly upregulated in the HCC tumors (Figure 1A). The immunohistochemical analysis of 79 pairs of HCC tissues and their corresponding adjacent tissues revealed that FOXP4 was primarily distributed in the cytoplasm, and its positive expression rate in HCC tissues was significantly higher than that in the corresponding adjacent tissues (Figure 1B and andC).C). Subsequently, TCGA database analysis using GEPIA revealed significantly higher FOXP4 expression in the HCC tissues than in normal liver tissues (Figure 1D). The OS of the patients with HCC was assessed using the Kaplan-Meier method based on FOXP4 and NDST2 expression. The patients were divided into two groups using the median cutoff value of standardized expression in HCC tissues. The patients with high FOXP4 expression exhibited poorer OS than those with low expression (P < 0.05) (Figure 1E).
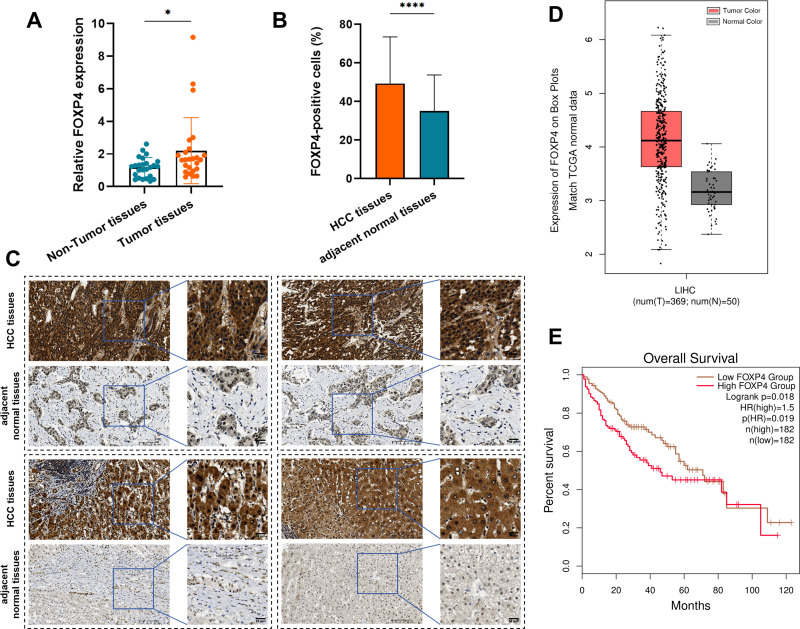
FOXP4 expression in HCC and its clinical implications. (A) Expression analysis of FOXP4 in 25 pairs of HCC tumor and adjacent non-cancerous tissue clinical samples (*P < 0.05). (B and C) The immunohistochemical staining analysis of FOXP4 revealed a markedly elevated expression level in HCC tissues compared to adjacent non-tumor tissues (****P < 0.0001). (D) Difference in FOXP4 expression levels between normal liver tissues and HCC tissues in TCGA database (P < 0.01). (E) The OS of clinical patients based on FOXP4 expression levels, where the patients with low FOXP4 expression had higher OS than those with high expression.
Molecular Expression and Significance of FOXP4 in Residual HCC After Incomplete Thermal Ablation
The CCK-8 experiment for analyzing HCC cell proliferation after heat stress revealed a significant enhancement in HCC cell proliferative capacity after heat stress (Figure 2A). The Transwell experiments revealed significantly increased migration and invasion abilities in the successfully constructed HCC cell heat stress model (Figure 2B and andC).C). The FOXP4 molecular and protein level changes in the heat-stressed HCC cells were examined using RT-qPCR and Western blotting. The RT-qPCR results demonstrated upregulated FOXP4 molecular expression in the heat-stressed HCC cells compared to the normal HCC cells (Figure 2D). Western blotting revealed a corresponding upregulation of FOXP4 protein level in the heat-stressed HCC cells (Figure 2E).
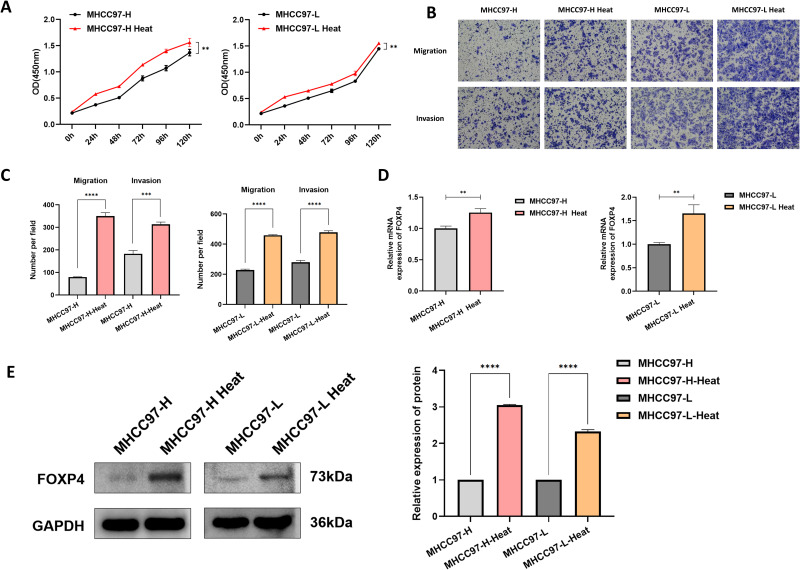
FOXP4 expression and significance in heat-stressed HCC cells. (A) CCK-8 assay demonstrating enhanced proliferative capacity of MHCC97-L and MHCC97-H cells after thermal ablation treatment had been simulated (**P < 0.01). (B and C) Transwell experiments detected increased migration and invasion abilities of MHCC97-L and MHCC97-H cells after thermal ablation treatment had been simulated (***P < 0.001, ****P < 0.0001, magnification ×100). (D) RT-qPCR experiment demonstrating elevated FOXP4 expression levels in MHCC97-L and MHCC97-H cells after heat stress compared to before heat stress (**P < 0.01). (E) Western blotting experiment detected increased FOXP4 protein expression levels in MHCC97-L and MHCC97-H cells after heat stress compared to before heat stress (****P < 0.0001).
Silencing the FOXP4 Gene Inhibits the Growth of Heat-Stressed HCC Cells
FOXP4 expression was silenced in the heat-stressed HCC cells, and RT-qPCR confirmed the effectiveness of this silencing (Figure 3A). Western blotting detection of protein levels after FOXP4 silencing demonstrated that FOXP4 protein levels were significantly reduced (Figure 3B). CCK-8 assays were conducted to explore the effect of silencing FOXP4 on the proliferative capacity of heat-stressed HCC cells. The assays revealed that suppressing FOXP4 significantly affected the growth of heat-stressed HCC cells (Figure 3C and andD).D). Subsequently, Transwell migration and invasion assays were performed to assess the influence of FOXP4 silencing on the migration and invasion abilities of heat-stressed HCC cells. Downregulating FOXP4 significantly suppressed the HCC cell migration and invasion abilities (Figure 3E and andF).F). Scratch assays were conducted to evaluate the effect of silencing FOXP4 on the migration ability of heat-stressed HCC cells, and they revealed that reducing FOXP4 significantly suppressed cell mobility (Figure 3G and andHH).
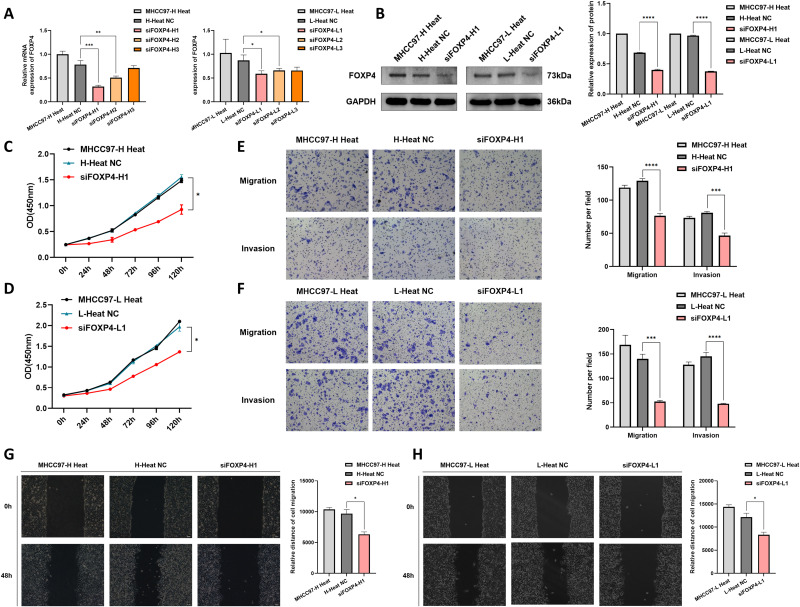
Detection of changes in gene expression and biological behavior after FOXP4 knockdown in heat-stressed MHCC97-L and MHCC97-H cells. (A) RT-qPCR detected decreased FOXP4 expression levels in heat-stressed HCC cells after FOXP4 knockdown (*P < 0.05, **P < 0.01, ***P < 0.001). (B) Western blotting experiments detected reduced FOXP4 protein expression levels in heat-stressed HCC cells after FOXP4 knockdown (****P < 0.0001). (C and D) CCK-8 assays detected weakened proliferative capacity of MHCC97-L and MHCC97-H cells after FOXP4 knockdown following heat stress (*P < 0.05). (E and F) Transwell experiments revealed reduced migration and invasion abilities of heat-stressed MHCC97-L and MHCC97-H cells after FOXP4 knockdown (***P < 0.001, ****P < 0.0001, magnification ×100). (G and H) The scratch assay detected decreased migration ability of heat-stressed MHCC97-L and MHCC97-H cells after FOXP4 knockdown (*P < 0.05, magnification ×100).
NDST2 is a Potential Target for Promoting Incomplete Residual HCC Progression by FOXP4
We analyzed the correlation between FOXP4 and NDST2 expression in liver tissue and liver cancer using the GEPIA database. The results demonstrated a moderate positive correlation between FOXP4 and NDST2 (Spearman = 0.62, Pearson = 0.54, P < 0.01) (Figure 4A). Subsequently, we performed CUT&Tag experiments to screen for downstream target genes of FOXP4 and evaluated the experimental quality and data characteristics (Figure 4B–E). Data analysis revealed that most FOXP4 signals were enriched in gene promoters (Figure 4E). FOXP4 reads were mainly enriched in the region from 1 kb upstream to 1 kb downstream of the transcription start site (TSS), in intronic regions, and in intergenic regions (Figure 4C). Furthermore, we visualized the peak detection results for NDST2 (Figure 4F) and observed positive strand peaks on the corresponding chromosome sequence of NDST2, indicating that NDST2 is a potential target for promoting incomplete residual HCC progression by FOXP4.
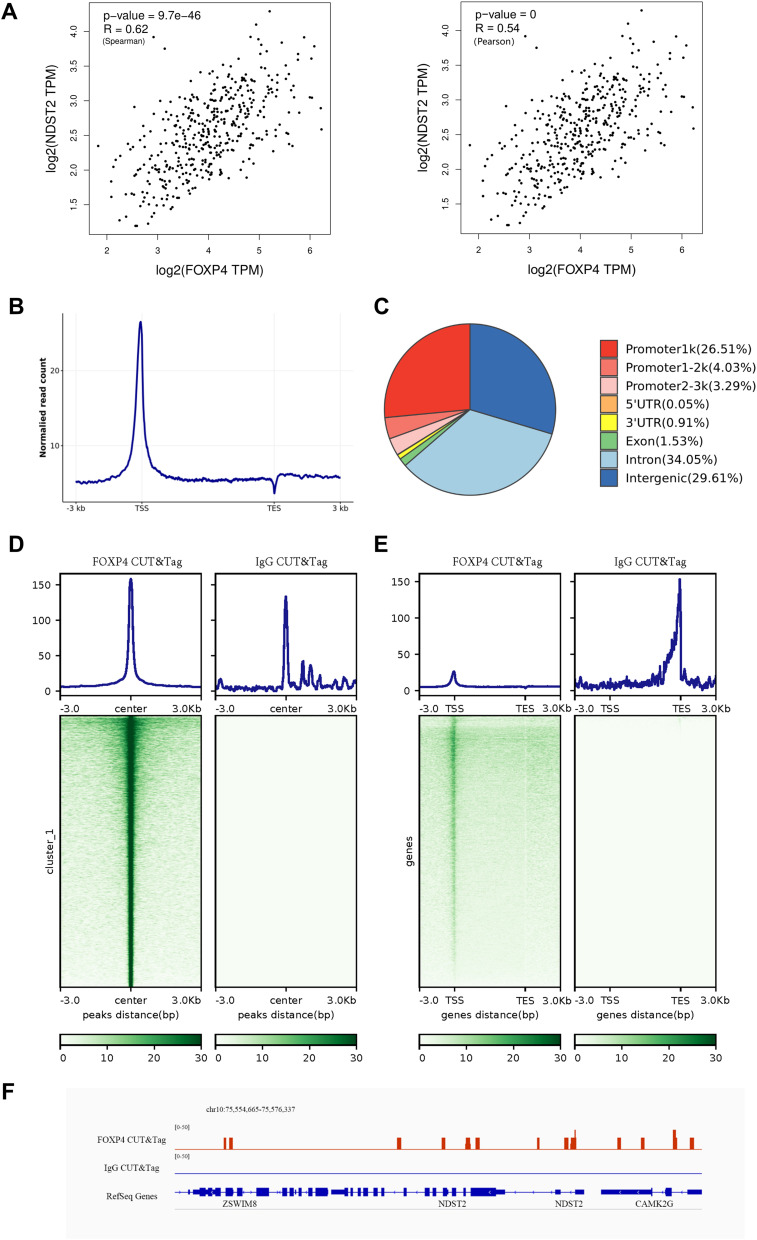
Assessment of the potential of NDST2 as a target for FOXP4 in promoting incomplete residual HCC progression. (A) Spearman’s and Pearson’s correlation analysis of FOXP4 and NDST2 expression in liver tissue and liver cancer. (B) Distribution of reads aligned to the genome in gene regions to assess the data quality and characteristics. TSS, transcription start site; TES, transcription end site. Strong signal peaks near the TSS indicate the enrichment of reads in that region. (C) Distribution of FOXP4 CUT&Tag peaks in genomic regions. (D) Signal enrichment at peak centers. A darker color in the center region of the peak indicates more concentrated signal enrichment. (E) Signal enrichment near genes. Most are concentrated near the TSS, indicating that gene reads are primarily enriched in that region. (F) Distribution of NDST2 sites relative to IgG promoters based on FOXP4 CUT&Tag analysis.
Expression and Significance of NDST2 in HCC Before and After Thermal Ablation
The GEPIA analysis of NDST2 differential expression between normal liver tissue and liver cancer tissue in the TCGA database revealed upregulated NDST2 in the liver cancer tissue compared to normal liver tissue (Figure 5A). Kaplan-Meier analysis of the OS of patients with HCC with different NDST2 levels revealed that the patients with high NDST2 expression exhibited a worse OS than those with low expression (P < 0.05) (Figure 5B). We further analyzed the changes in NDST2 expression in HCC cells after heat stress. RT-qPCR experiments were performed to detect changes in gene expression, and Western blotting experiments were conducted to measure protein levels. The experimental outcomes demonstrated that NDST2 gene and protein levels were upregulated in heat-stressed HCC cells compared to normal cancer cells (Figure 5C and andDD).
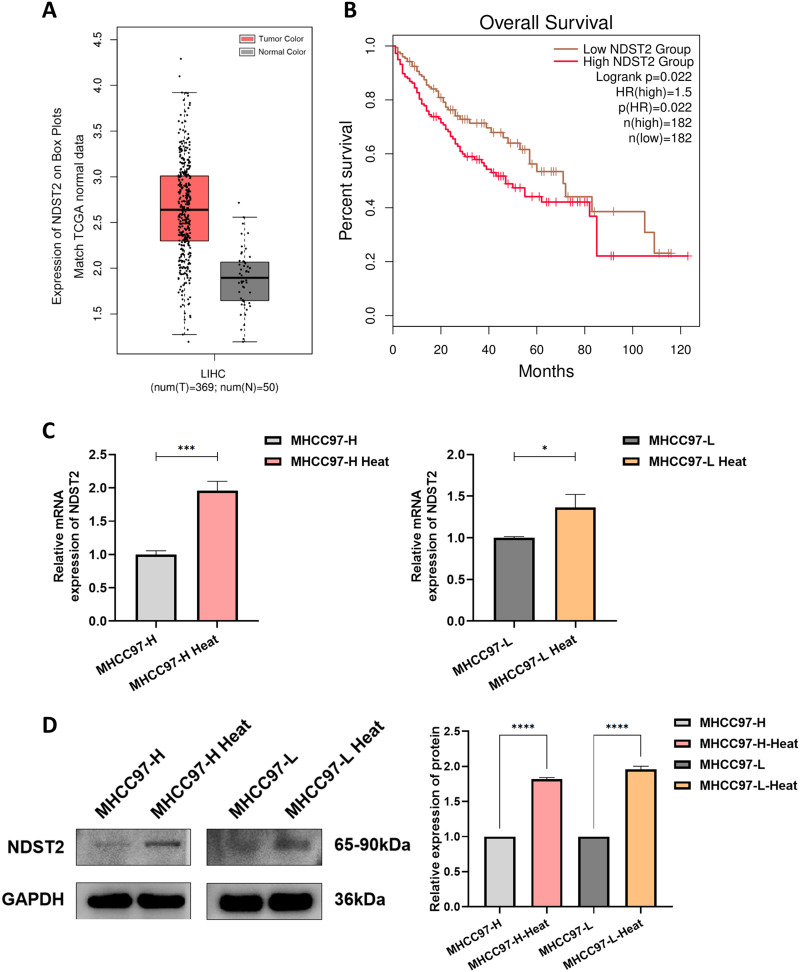
NDST2 expression and significance in HCC before and after thermal ablation. (A) Differential expression of NDST2 in normal liver tissue and liver cancer tissue in TCGA database (P < 0.01). (B) The OS of clinical patients was assessed based on the NDST2 expression levels. The low-expression group demonstrated a higher OS rate than the high-expression group. (C) RT-qPCR demonstrating increased NDST2 expression in heat-stressed MHCC97-L and MHCC97-H cells compared to before heat stress (*P < 0.05, ***P < 0.001). (D) Western blotting detecting increased NDST2 protein expression in heat-stressed MHCC97-L and MHCC97-H cells compared to before heat stress (****P < 0.0001).
Silencing the NDST2 Gene Inhibits the Growth of Heat-Stressed HCC Cells
The siRNA transfection reagent with the most significant knockdown effect was selected through RT-qPCR experiments (Figure 6A). NDST2 protein levels were validated using Western blotting analysis, demonstrating decreased NDST2 protein levels in heat-stressed HCC cells following NDST2 knockdown (Figure 6B). The CCK-8 assay demonstrated that suppressing NDST2 significantly impeded the proliferative ability of heat-stressed HCC cells (Figure 6C and andD).D). The Transwell experiment indicated that the migration and invasion abilities of heat-stressed HCC cells were suppressed after NDST2 knockdown (Figure 6E and andF).F). In addition, the scratch experiment revealed that downregulating NDST2 expression significantly inhibited the migration capacity of HCC cells (Figure 6G and andHH).
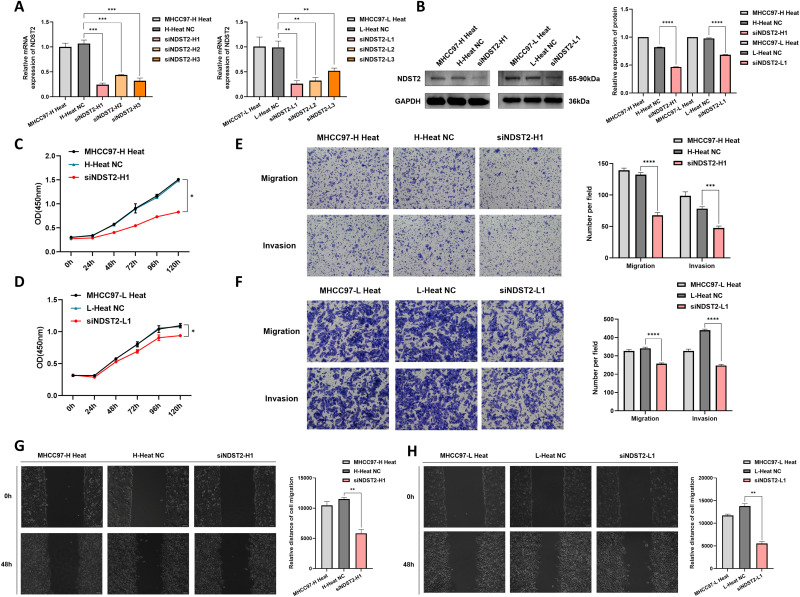
Examination of gene expression and changes in biological behavior after NDST2 knockdown in heat-stressed MHCC97-L and MHCC97-H HCC cells. (A) RT-qPCR experiment detected decreased NDST2 expression in heat-stressed HCC cells after NDST2 knockdown (**P < 0.01, ***P < 0.001). (B) Western blotting experiment detected reduced NDST2 protein expression in heat-stressed HCC cells after NDST2 knockdown (****P < 0.0001). (C and D) CCK-8 experiment revealing the weakened proliferation capacity of heat-stressed MHCC97-L and MHCC97-H cells after NDST2 knockdown (*P < 0.05). (E and F) Transwell assays detected decreased migration and invasion ability of heat-stressed MHCC97-L and MHCC97-H cells after NDST2 knockdown (***P < 0.001, ****P < 0.0001, magnification ×100). (G and H) The scratch assay detected the weakened migration ability of heat-stressed MHCC97-L and MHCC97-H cells after NDST2 knockdown (**P < 0.01, magnification ×100).
FOXP4 Regulates the Progression of Incomplete Residual HCC After Thermal Ablation Through NDST2
To elucidate the interaction between FOXP4 and NDST2, we evaluated the NDST2 gene and protein expression levels in a heat-stressed HCC cell line with FOXP4 knockdown. RT-qPCR (Figure 7A) revealed reduced NDST2 molecular expression levels in the heat-stressed HCC cells following FOXP4 knockdown. Similarly, Western blotting (Figure 7B) demonstrated reduced NDST2 protein expression levels in heat-stressed HCC cells with FOXP4 knockdown.
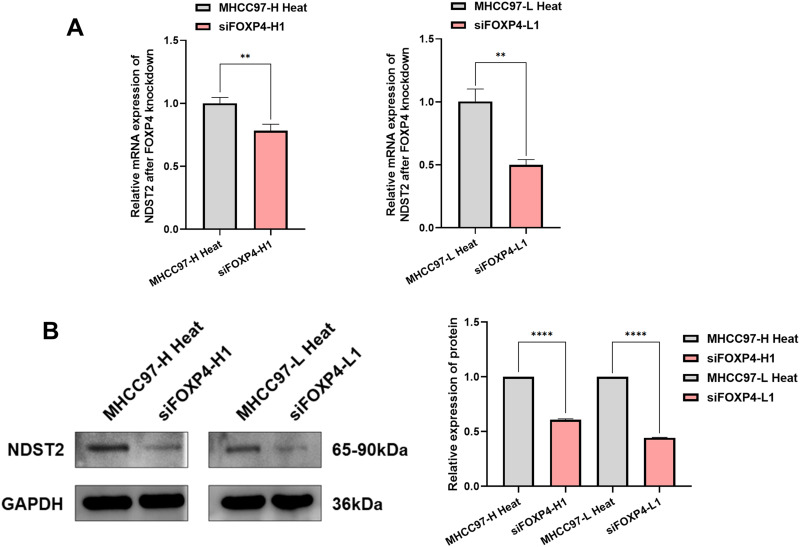
Examination of NDST2 expression in heat-stressed HCC cells with FOXP4 knockdown. (A) RT-qPCR experiment detected decreased NDST2 expression levels in heat-stressed HCC cells after FOXP4 knockdown (**P < 0.01). (B) Western blotting experiment detected reduced NDST2 protein expression levels after FOXP4 knockdown in heat-stressed HCC cells (****P < 0.0001).
Discussion
Liver cancer is a common malignant tumor with increasing incidence and mortality rates, posing a serious threat to human health.27 Thermal ablation is a widely used local treatment method for managing liver cancer. However, the issue of incomplete thermal ablation persists, which might lead to tumor recurrence and metastasis, affecting prognosis.28 Gaining a profound understanding of the molecular mechanisms driving the progression of residual cancer cells post-thermal ablation is essential to reduce the risk of recurrence and enhance prognosis.
Prior research established a significant association between the FOXP4 gene and HCC onset and progression, where elevated FOXP4 expression is strongly linked to tumor invasion and metastasis.29 The literature suggested that downregulating FOXP4 inhibits tumor proliferation and metastasis and that FOXP4 is a target gene to regulate tumor occurrence and development.30–32 However, few studies have linked FOXP4 to incomplete thermal ablation in residual HCC, and whether it and its regulatory network can become a target controlling the biological progression of residual HCC after incomplete thermal ablation has not been clearly reported. In this study, we investigated the variance in FOXP4 expression between liver cancer tissue and non-cancerous liver tissue using clinical samples and big data analysis. We validated the association between high FOXP4 expression and HCC occurrence and development. The data results demonstrated upregulated FOXP4 expression in liver cancer tissue compared to non-tumor tissue and its correlation with poor prognosis in patients with HCC. Using an in vitro incomplete ablation cell model, we confirmed the high FOXP4 expression in residual HCC cells through RT-qPCR and Western blotting. Subsequently, we downregulated FOXP4 expression in heat-stressed HCC cells to explore the effect of FOXP4 on the biological progression of heat-stressed HCC cells. The in vitro cell experiments revealed markedly decreased proliferation, invasion, and migration capabilities of the heat-stressed HCC cells following FOXP4 knockdown. These findings demonstrated the tumor-suppressive function of FOXP4 expression down-regulation in regulating the progression and apoptosis of residual HCC cells following incomplete thermal ablation.
NDST2 is an enzyme that catalyzes sulfation modification and regulates the HS content in cells and tissues by increasing the length of the HS chains.33 Heparin and heparin-like molecules exhibit specific effects on tumor initiation and progression by inhibiting tumor cell proliferation and growth, lowering the likelihood of tumor formation and metastasis.34 Previously, we investigated the relationship between NDST2 and HCC and identified a close association between high NDST2 expression and accelerated progression of heat-stressed HCC cells.22 In this study, TCGA clinical data analysis revealed differential expression of NDST2 between normal liver tissue and liver cancer tissue, with increased expression in liver cancer tissue. The Kaplan-Meier method revealed that patients with high NDST2 expression exhibited a poorer OS than those with low expression. Similarly, we used an in vitro cell model simulating incomplete ablation HCC cells to detect changes in NDST2 expression in heat-stressed HCC cells. The results demonstrated upregulation at the gene and protein levels. We downregulated NDST2 expression in heat-stressed HCC cells and explored its effect on the biological progression of heat-stressed HCC cells. Similar to FOXP4, the biological behavior of heat-stressed HCC cells was significantly weakened after NDST2 knockdown.
We performed validation using big data analysis, in vitro cell experiments, and CUT&Tag experiments to delve deeper into the potential regulatory connection between FOXP4 and NDST2. Spearman and Pearson correlation analyses were used to explore the expression correlation between FOXP4 and NDST2 in normal liver and liver cancer. The experiment revealed a moderately positive correlation between FOXP4 and NDST2. CUT&Tag experiments were performed to identify downstream target genes of FOXP4, and the chromosomal sequence corresponding to NDST2 was visualized, exhibiting positive peaks on the sense strand and suggesting that NDST2 might be a potential target of FOXP4. Finally, RT-qPCR and Western blotting were conducted to measure the NDST2 gene and protein expression levels in heat-stressed HCC cells after FOXP4 had been knocked down. The results demonstrated reduced NDST2 gene and protein expression levels in heat-stressed HCC cells following FOXP4 knockdown. These findings support the important roles of FOXP4 and NDST2 in the biological progression of residual cancer cells following incomplete ablation, suggesting that FOXP4 might regulate the biological progression of residual HCC post-incomplete ablation via NDST2.
The study also has some limitations. Firstly, the study investigated the roles of FOXP4 and NDST2 genes in the rapid progression of residual cancer following incomplete thermal ablation and the potential association between them. However, the precise mechanisms by which FOXP4 mediates the involvement of NDST2 in heat-stressed residual HCC remain largely unknown and require further research. Our findings, while suggestive, are limited by the scarcity of comprehensive molecular investigations, especially those about cell signal pathway analysis, such as dual-luciferase assays, rescue experiments, and ChIP-seq experiments. Consequently, we intend to conduct more extensive research in the future to fully understand the complex interplay between FOXP4 and NDST2 in heat-stressed residual HCC progression. Secondly, while our study has provided preliminary evidence of a close correlation between high expression of two genes (NDST2 and FOXP4) and accelerated progression of heat-stressed HCC cells, it is important to note that we have not thoroughly examined the potential role of both of them in other pathways. The potential interactions between NDST2 and FOXP4 across diverse biological pathways necessitate more detailed investigation and consideration.
Conclusion
We identified a novel pathway, the FOXP4/NDST2 axis, which regulates the rapid progression of residual HCC following incomplete ablation. FOXP4 potentially influenced the biological characteristics of residual HCC post-incomplete ablation, triggering rapid progression by controlling NDST2 expression, thereby affecting the prognosis of patients with HCC. Our study offers new insights and approaches for devising treatment strategies and preventive measures for prognosis in HCC thermal ablation.
Acknowledgments
Thanks to the Key Laboratory of Ultrasonic Molecular Imaging and Artificial Intelligence, Guangxi Zhuang Autonomous Region Engineering Research Center for Artificial Intelligence Analysis of Multimodal Tumor Images, Guangxi Key Laboratory of Early Prevention and Treatment for Regional High Frequency Tumor, and Key Laboratory of Early Prevention and Treatment for Regional High Frequency Tumor (Guangxi Medical University), Ministry of Education for their support of this study. We thank Home for Researchers editorial team (www.home-for-researchers.com) for language editing service.
Funding Statement
This study was funded by the National Natural Science Foundation of China (82160350, 81960329, 82160336), the Natural Science Foundation of Guangxi (2020GXNSFDA238005, 2023JJB140557), and the China Postdoctoral Science Foundation (2023M744191).
Abbreviations
CCK-8, Cell Counting Kit-8; CUT&Tag, Cleavage Under Targets and Tagmentation; NDST2, N-deacetylase and N-sulfotransferase 2; EMT, Epithelial-mesenchymal transition; FOXP4: Forkhead box P4; GEPIA, Gene Expression Profiling Interactive Analysis; HCC, Hepatocellular carcinoma; HS, Heparan sulfate; LncRNA, Long non-coding RNA; MiR, MicroRNA; OS, Overall survival; RT-qPCR, quantitative real-time PCR; siRNA, Small interfering RNA; SD, standard deviation; TCGA, The Cancer Genome Atlas.
Data Sharing Statement
All data generated or analyzed during this study are included in the paper and its additional files.
Ethics Approval and Informed Consent
Ethical approval for this study (NO.2019-KY-(084)) was provided by the Ethical Committee of The First Affiliated Hospital of Guangxi Medical University. All research was conducted in accordance with both the Declarations of Helsinki and Istanbul.
References
Articles from Journal of Hepatocellular Carcinoma are provided here courtesy of Dove Press
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Exploring the impact of insufficient thermal ablation on hepatocellular carcinoma: NDST2 overexpression mechanism and its role in facilitating growth and invasion of residual cancer cells.
Int J Hyperthermia, 41(1):2353309, 15 May 2024
Cited by: 1 article | PMID: 38749506
Long non-coding RNA FOXP4-AS1 facilitates the biological functions of hepatocellular carcinoma cells via downregulating ZC3H12D by mediating H3K27me3 through recruitment of EZH2.
Cell Biol Toxicol, 38(6):1047-1062, 21 Sep 2021
Cited by: 7 articles | PMID: 34545456 | PMCID: PMC9750913
RAD21: A Key Transcriptional Regulator in the Development of Residual Liver Cancer.
J Hepatocell Carcinoma, 11:285-304, 05 Feb 2024
Cited by: 0 articles | PMID: 38344425 | PMCID: PMC10854404
Long Noncoding RNA FOXP4-AS1 Predicts Unfavourable Prognosis and Regulates Proliferation and Invasion in Hepatocellular Carcinoma.
Biomed Res Int, 2021:8850656, 01 Feb 2021
Cited by: 6 articles | PMID: 33604387 | PMCID: PMC7870313



