Abstract
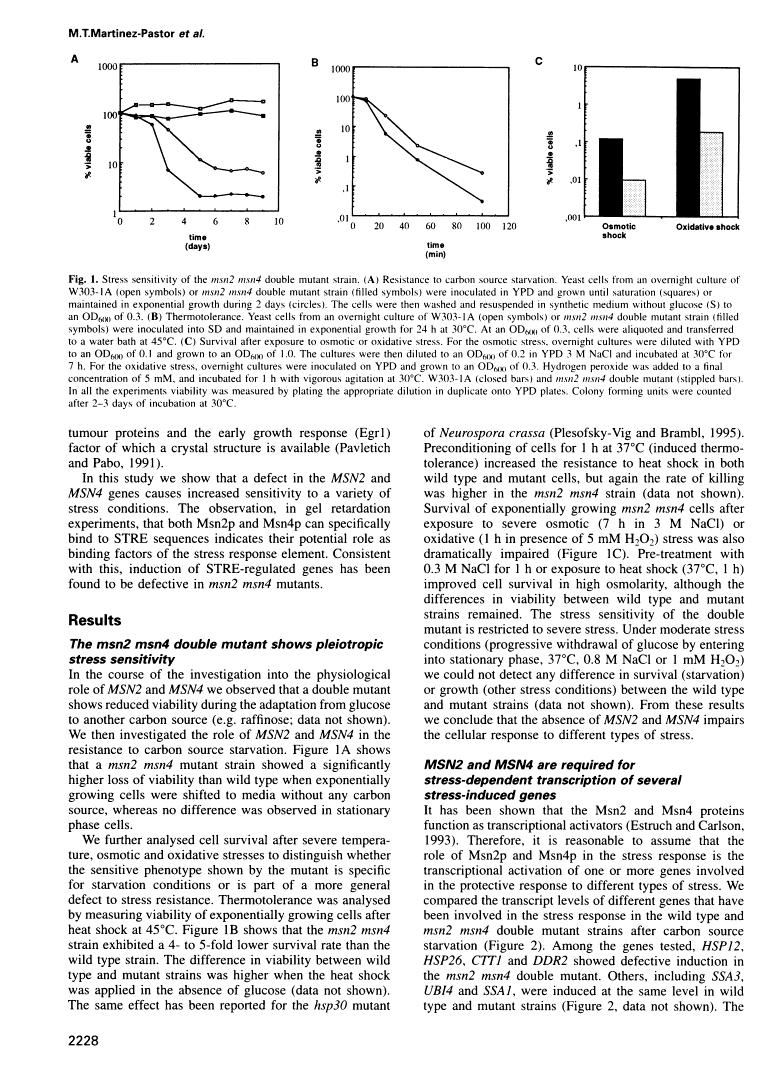
Free full text

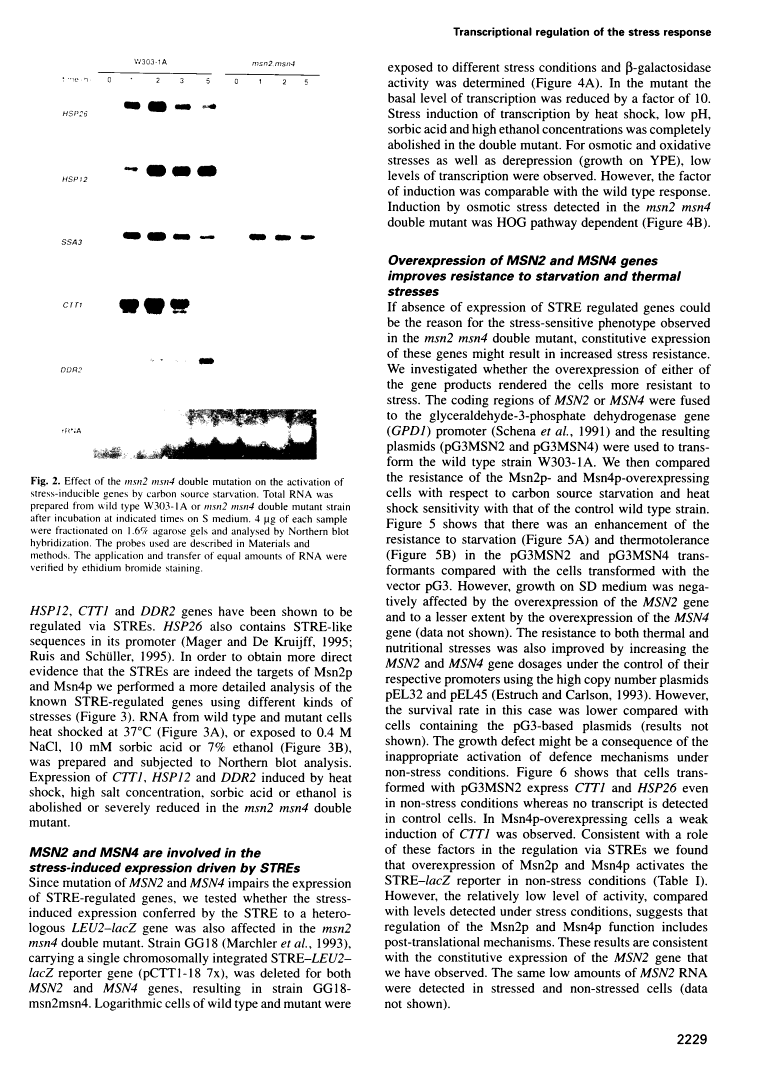
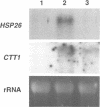
The Saccharomyces cerevisiae zinc finger proteins Msn2p and Msn4p are required for transcriptional induction through the stress response element (STRE).
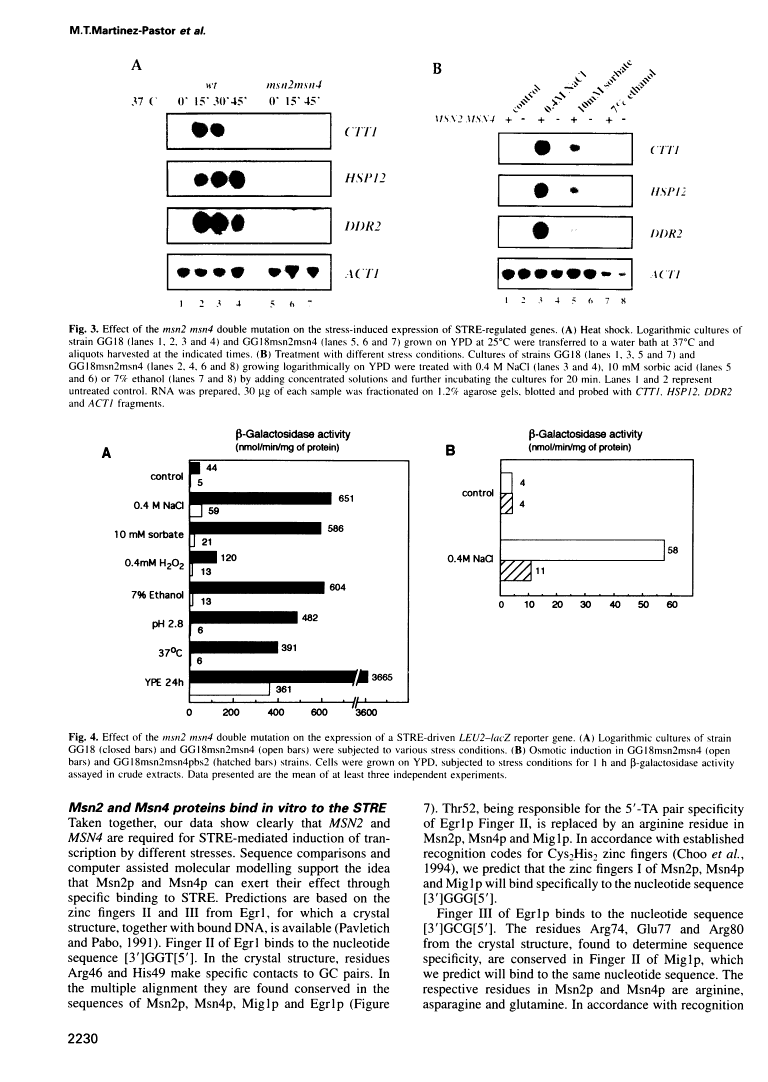
Abstract
The MSN2 and MSN4 genes encode homologous and functionally redundant Cys2His2 zinc finger proteins. A disruption of both MSN2 and MSN4 genes results in a higher sensitivity to different stresses, including carbon source starvation, heat shock and severe osmotic and oxidative stresses. We show that MSN2 and MSN4 are required for activation of several yeast genes such as CTT1, DDR2 and HSP12, whose induction is mediated through stress-response elements (STREs). Msn2p and Msn4p are important factors for the stress-induced activation of STRE dependent promoters and bind specifically to STRE-containing oligonucleotides. Our results suggest that MSN2 and MSN4 encode a DNA-binding component of the stress responsive system and it is likely that they act as positive transcription factors.
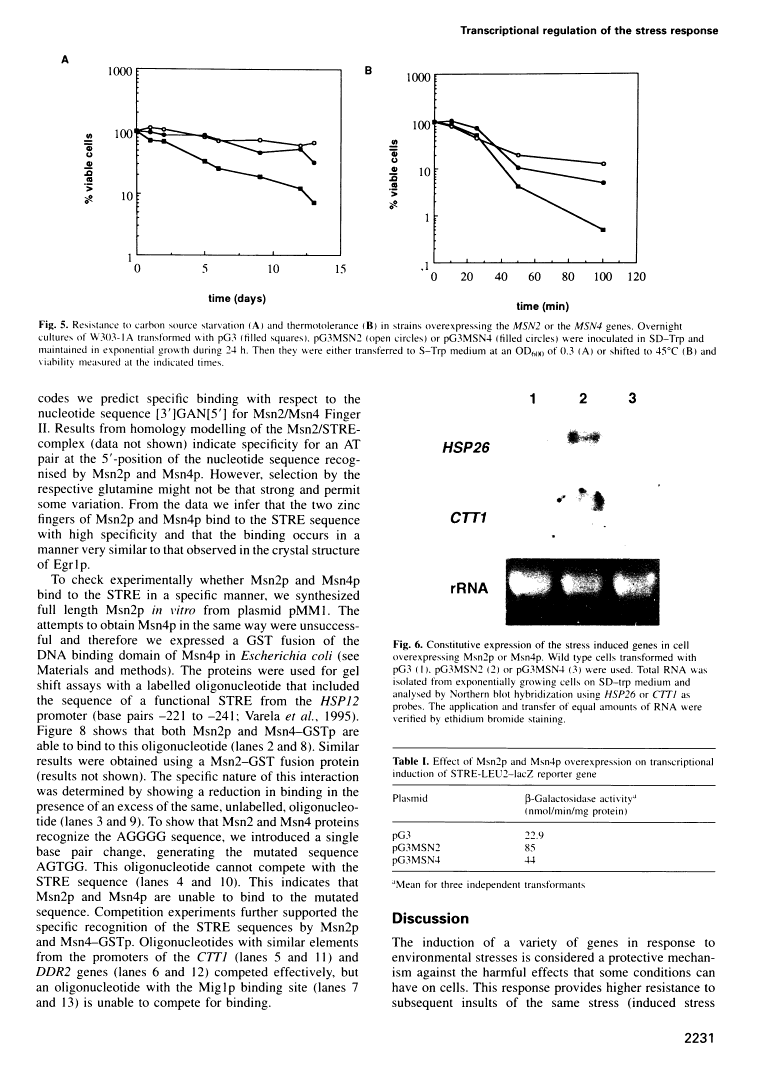
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.9M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ammerer G. Sex, stress and integrity: the importance of MAP kinases in yeast. Curr Opin Genet Dev. 1994 Feb;4(1):90–95. [Abstract] [Google Scholar]
- Bienz M, Pelham HR. Mechanisms of heat-shock gene activation in higher eukaryotes. Adv Genet. 1987;24:31–72. [Abstract] [Google Scholar]
- Boorstein WR, Craig EA. Regulation of a yeast HSP70 gene by a cAMP responsive transcriptional control element. EMBO J. 1990 Aug;9(8):2543–2553. [Europe PMC free article] [Abstract] [Google Scholar]
- Brewster JL, de Valoir T, Dwyer ND, Winter E, Gustin MC. An osmosensing signal transduction pathway in yeast. Science. 1993 Mar 19;259(5102):1760–1763. [Abstract] [Google Scholar]
- Choo Y, Klug A. Selection of DNA binding sites for zinc fingers using rationally randomized DNA reveals coded interactions. Proc Natl Acad Sci U S A. 1994 Nov 8;91(23):11168–11172. [Europe PMC free article] [Abstract] [Google Scholar]
- Choo Y, Sánchez-García I, Klug A. In vivo repression by a site-specific DNA-binding protein designed against an oncogenic sequence. Nature. 1994 Dec 15;372(6507):642–645. [Abstract] [Google Scholar]
- Estruch F, Carlson M. Increased dosage of the MSN1 gene restores invertase expression in yeast mutants defective in the SNF1 protein kinase. Nucleic Acids Res. 1990 Dec 11;18(23):6959–6964. [Europe PMC free article] [Abstract] [Google Scholar]
- Estruch F, Carlson M. Two homologous zinc finger genes identified by multicopy suppression in a SNF1 protein kinase mutant of Saccharomyces cerevisiae. Mol Cell Biol. 1993 Jul;13(7):3872–3881. [Europe PMC free article] [Abstract] [Google Scholar]
- Gallwitz D, Sures I. Structure of a split yeast gene: complete nucleotide sequence of the actin gene in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1980 May;77(5):2546–2550. [Europe PMC free article] [Abstract] [Google Scholar]
- Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994 Aug 5;265(5173):808–811. [Abstract] [Google Scholar]
- Herskowitz I. MAP kinase pathways in yeast: for mating and more. Cell. 1995 Jan 27;80(2):187–197. [Abstract] [Google Scholar]
- Kamada Y, Jung US, Piotrowski J, Levin DE. The protein kinase C-activated MAP kinase pathway of Saccharomyces cerevisiae mediates a novel aspect of the heat shock response. Genes Dev. 1995 Jul 1;9(13):1559–1571. [Abstract] [Google Scholar]
- Kobayashi N, McEntee K. Evidence for a heat shock transcription factor-independent mechanism for heat shock induction of transcription in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6550–6554. [Europe PMC free article] [Abstract] [Google Scholar]
- Kobayashi N, McEntee K. Identification of cis and trans components of a novel heat shock stress regulatory pathway in Saccharomyces cerevisiae. Mol Cell Biol. 1993 Jan;13(1):248–256. [Europe PMC free article] [Abstract] [Google Scholar]
- Kuge S, Jones N. YAP1 dependent activation of TRX2 is essential for the response of Saccharomyces cerevisiae to oxidative stress by hydroperoxides. EMBO J. 1994 Feb 1;13(3):655–664. [Europe PMC free article] [Abstract] [Google Scholar]
- Kyriakis JM, Banerjee P, Nikolakaki E, Dai T, Rubie EA, Ahmad MF, Avruch J, Woodgett JR. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994 May 12;369(6476):156–160. [Abstract] [Google Scholar]
- Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. [Abstract] [Google Scholar]
- Mager WH, Ferreira PM. Stress response of yeast. Biochem J. 1993 Feb 15;290(Pt 1):1–13. [Europe PMC free article] [Abstract] [Google Scholar]
- Mager WH, De Kruijff AJ. Stress-induced transcriptional activation. Microbiol Rev. 1995 Sep;59(3):506–531. [Europe PMC free article] [Abstract] [Google Scholar]
- Marchler G, Schüller C, Adam G, Ruis H. A Saccharomyces cerevisiae UAS element controlled by protein kinase A activates transcription in response to a variety of stress conditions. EMBO J. 1993 May;12(5):1997–2003. [Europe PMC free article] [Abstract] [Google Scholar]
- McClanahan T, McEntee K. DNA damage and heat shock dually regulate genes in Saccharomyces cerevisiae. Mol Cell Biol. 1986 Jan;6(1):90–96. [Europe PMC free article] [Abstract] [Google Scholar]
- Morimoto RI, Sarge KD, Abravaya K. Transcriptional regulation of heat shock genes. A paradigm for inducible genomic responses. J Biol Chem. 1992 Nov 5;267(31):21987–21990. [Abstract] [Google Scholar]
- Pavletich NP, Pabo CO. Zinc finger-DNA recognition: crystal structure of a Zif268-DNA complex at 2.1 A. Science. 1991 May 10;252(5007):809–817. [Abstract] [Google Scholar]
- Piper PW. Molecular events associated with acquisition of heat tolerance by the yeast Saccharomyces cerevisiae. FEMS Microbiol Rev. 1993 Aug;11(4):339–355. [Abstract] [Google Scholar]
- Praekelt UM, Meacock PA. HSP12, a new small heat shock gene of Saccharomyces cerevisiae: analysis of structure, regulation and function. Mol Gen Genet. 1990 Aug;223(1):97–106. [Abstract] [Google Scholar]
- Rose M, Botstein D. Construction and use of gene fusions to lacZ (beta-galactosidase) that are expressed in yeast. Methods Enzymol. 1983;101:167–180. [Abstract] [Google Scholar]
- Rothstein RJ. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. [Abstract] [Google Scholar]
- Rouse J, Cohen P, Trigon S, Morange M, Alonso-Llamazares A, Zamanillo D, Hunt T, Nebreda AR. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994 Sep 23;78(6):1027–1037. [Abstract] [Google Scholar]
- Ruis H, Schüller C. Stress signaling in yeast. Bioessays. 1995 Nov;17(11):959–965. [Abstract] [Google Scholar]
- Schena M, Picard D, Yamamoto KR. Vectors for constitutive and inducible gene expression in yeast. Methods Enzymol. 1991;194:389–398. [Abstract] [Google Scholar]
- Schüller C, Brewster JL, Alexander MR, Gustin MC, Ruis H. The HOG pathway controls osmotic regulation of transcription via the stress response element (STRE) of the Saccharomyces cerevisiae CTT1 gene. EMBO J. 1994 Sep 15;13(18):4382–4389. [Europe PMC free article] [Abstract] [Google Scholar]
- Smith DB, Johnson KS. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. [Abstract] [Google Scholar]
- Spevak W, Fessl F, Rytka J, Traczyk A, Skoneczny M, Ruis H. Isolation of the catalase T structural gene of Saccharomyces cerevisiae by functional complementation. Mol Cell Biol. 1983 Sep;3(9):1545–1551. [Europe PMC free article] [Abstract] [Google Scholar]
- Susek RE, Lindquist S. Transcriptional derepression of the Saccharomyces cerevisiae HSP26 gene during heat shock. Mol Cell Biol. 1990 Dec;10(12):6362–6373. [Europe PMC free article] [Abstract] [Google Scholar]
- Tamai KT, Liu X, Silar P, Sosinowski T, Thiele DJ. Heat shock transcription factor activates yeast metallothionein gene expression in response to heat and glucose starvation via distinct signalling pathways. Mol Cell Biol. 1994 Dec;14(12):8155–8165. [Europe PMC free article] [Abstract] [Google Scholar]
- Thiele DJ. Metal-regulated transcription in eukaryotes. Nucleic Acids Res. 1992 Mar 25;20(6):1183–1191. [Europe PMC free article] [Abstract] [Google Scholar]
- Thomas BJ, Rothstein R. Elevated recombination rates in transcriptionally active DNA. Cell. 1989 Feb 24;56(4):619–630. [Abstract] [Google Scholar]
- Varela JC, Praekelt UM, Meacock PA, Planta RJ, Mager WH. The Saccharomyces cerevisiae HSP12 gene is activated by the high-osmolarity glycerol pathway and negatively regulated by protein kinase A. Mol Cell Biol. 1995 Nov;15(11):6232–6245. [Europe PMC free article] [Abstract] [Google Scholar]
- Werner-Washburne M, Stone DE, Craig EA. Complex interactions among members of an essential subfamily of hsp70 genes in Saccharomyces cerevisiae. Mol Cell Biol. 1987 Jul;7(7):2568–2577. [Europe PMC free article] [Abstract] [Google Scholar]
- Wieser R, Adam G, Wagner A, Schüller C, Marchler G, Ruis H, Krawiec Z, Bilinski T. Heat shock factor-independent heat control of transcription of the CTT1 gene encoding the cytosolic catalase T of Saccharomyces cerevisiae. J Biol Chem. 1991 Jul 5;266(19):12406–12411. [Abstract] [Google Scholar]
- Wu AL, Moye-Rowley WS. GSH1, which encodes gamma-glutamylcysteine synthetase, is a target gene for yAP-1 transcriptional regulation. Mol Cell Biol. 1994 Sep;14(9):5832–5839. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from The EMBO Journal are provided here courtesy of Nature Publishing Group
Full text links
Read article at publisher's site: https://doi.org/10.1002/j.1460-2075.1996.tb00576.x
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc450147?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1002/j.1460-2075.1996.tb00576.x
Article citations
Conserved signaling modules regulate filamentous growth in fungi: a model for eukaryotic cell differentiation.
Genetics, 228(2):iyae122, 01 Oct 2024
Cited by: 0 articles | PMID: 39239926
Review
Toxicants improve glycerol production in the fermentation of undetoxified hydrolysate by Candida glycerinogenes.
Biotechnol Lett, 46(6):1057-1068, 31 Jul 2024
Cited by: 0 articles | PMID: 39085486
Natural variation in yeast reveals multiple paths for acquiring higher stress resistance.
BMC Biol, 22(1):149, 04 Jul 2024
Cited by: 0 articles | PMID: 38965504 | PMCID: PMC11225312
An adaptive biomolecular condensation response is conserved across environmentally divergent species.
Nat Commun, 15(1):3127, 11 Apr 2024
Cited by: 5 articles | PMID: 38605014 | PMCID: PMC11009240
A novel natural variation in the promoter of GmCHX1 regulates conditional gene expression to improve salt tolerance in soybean.
J Exp Bot, 75(3):1051-1062, 01 Feb 2024
Cited by: 2 articles | PMID: 37864556 | PMCID: PMC10837011
Go to all (625) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Msn2p, a zinc finger DNA-binding protein, is the transcriptional activator of the multistress response in Saccharomyces cerevisiae.
Proc Natl Acad Sci U S A, 93(12):5777-5782, 01 Jun 1996
Cited by: 289 articles | PMID: 8650168 | PMCID: PMC39137
Zinc starvation induces a stress response in Saccharomyces cerevisiae that is mediated by the Msn2p and Msn4p transcriptional activators.
FEMS Yeast Res, 9(8):1187-1195, 31 Jul 2009
Cited by: 7 articles | PMID: 19702872
Elevated expression of genes under the control of stress response element (STRE) and Msn2p in an ethanol-tolerance sake yeast Kyokai no. 11.
J Biosci Bioeng, 104(3):163-170, 01 Sep 2007
Cited by: 28 articles | PMID: 17964478
[Alternative ways of stress regulation in cells of Saccharomyces cerevisiae: transcriptional activators Msn2 and Msn4].
Tsitologiia, 51(3):271-278, 01 Jan 2009
Cited by: 1 article | PMID: 19435282
Review