Abstract
Free full text

The genes for tumor necrosis factor (TNF-alpha) and lymphotoxin (TNF-beta) are tandemly arranged on chromosome 17 of the mouse.
Abstract
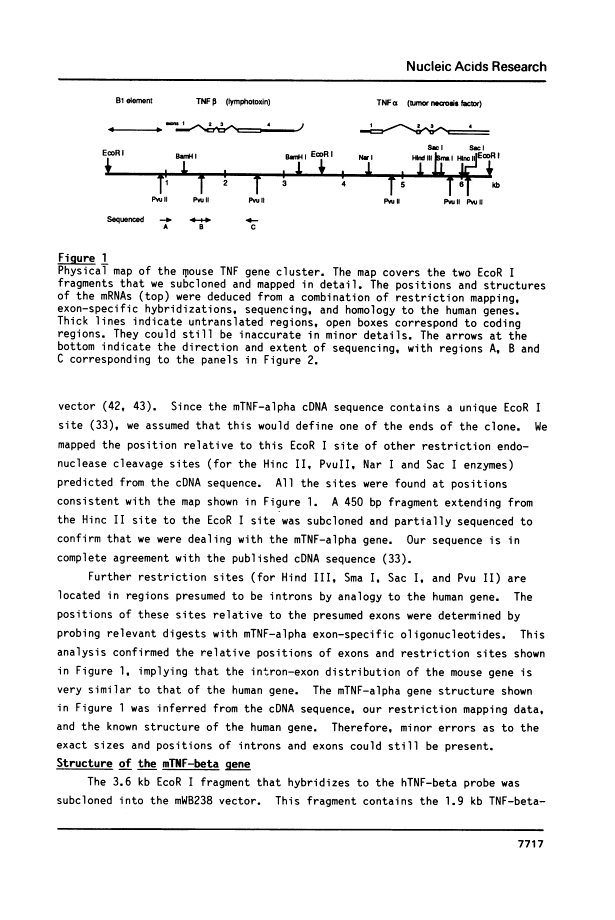
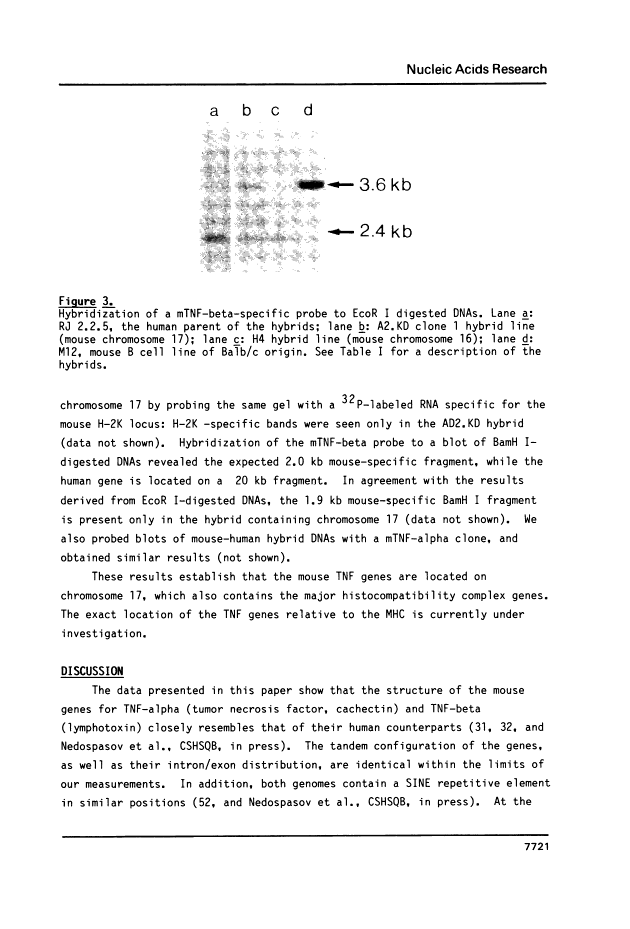
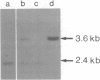
We have isolated clones containing the gene for tumor necrosis factor (TNF-alpha) from a mouse genomic library. Four out of five clones containing the TNF-alpha gene also hybridized to a human lymphotoxin (TNF-beta) probe. We constructed a restriction enzyme cleavage map of a 6.4 kb region from one of the genomic clones. From partial sequencing data and hybridizations with exon-specific oligonucleotide probes, we conclude that this region contains the mouse TNF-alpha and TNF-beta genes in a tandem arrangement, that they are separated by only about 1100 bases, and that their intron-exon structure is very similar to that seen in man. We probed genomic blots of DNA from human/mouse hybrids containing single mouse chromosomes for the presence of the mouse TNF genes. The results show that the genes are located on mouse chromosome 17, which also contains the major histocompatibility complex. Therefore, both the mouse and the human TNF genes are tandemly arranged and located on the same chromosome as the MHC.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.0M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Granger GA, Williams TW. Lymphocyte cytotoxicity in vitro: activation and release of a cytotoxic factor. Nature. 1968 Jun 29;218(5148):1253–1254. [Abstract] [Google Scholar]
- Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. [Europe PMC free article] [Abstract] [Google Scholar]
- Kull FC, Jr, Cuatrecasas P. Preliminary characterization of the tumor cell cytotoxin in tumor necrosis serum. J Immunol. 1981 Apr;126(4):1279–1283. [Abstract] [Google Scholar]
- Ruddle NH, Powell MB, Conta BS. Lymphotoxin, a biologically relevant model lymphokine. Lymphokine Res. 1983;2(1):23–31. [Abstract] [Google Scholar]
- Old LJ. Tumor necrosis factor (TNF). Science. 1985 Nov 8;230(4726):630–632. [Abstract] [Google Scholar]
- Sugarman BJ, Aggarwal BB, Hass PE, Figari IS, Palladino MA, Jr, Shepard HM. Recombinant human tumor necrosis factor-alpha: effects on proliferation of normal and transformed cells in vitro. Science. 1985 Nov 22;230(4728):943–945. [Abstract] [Google Scholar]
- Kelker HC, Oppenheim JD, Stone-Wolff D, Henriksen-DeStefano D, Aggarwal BB, Stevenson HC, Vilcek J. Characterization of human tumor necrosis factor produced by peripheral blood monocytes and its separation from lymphotoxin. Int J Cancer. 1985 Jul 15;36(1):69–73. [Abstract] [Google Scholar]
- Nedwin GE, Svedersky LP, Bringman TS, Palladino MA, Jr, Goeddel DV. Effect of interleukin 2, interferon-gamma, and mitogens on the production of tumor necrosis factors alpha and beta. J Immunol. 1985 Oct;135(4):2492–2497. [Abstract] [Google Scholar]
- Beutler B, Greenwald D, Hulmes JD, Chang M, Pan YC, Mathison J, Ulevitch R, Cerami A. Identity of tumour necrosis factor and the macrophage-secreted factor cachectin. Nature. 1985 Aug 8;316(6028):552–554. [Abstract] [Google Scholar]
- Beutler B, Cerami A. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature. 1986 Apr 17;320(6063):584–588. [Abstract] [Google Scholar]
- Beutler B, Mahoney J, Le Trang N, Pekala P, Cerami A. Purification of cachectin, a lipoprotein lipase-suppressing hormone secreted by endotoxin-induced RAW 264.7 cells. J Exp Med. 1985 May 1;161(5):984–995. [Europe PMC free article] [Abstract] [Google Scholar]
- Torti FM, Dieckmann B, Beutler B, Cerami A, Ringold GM. A macrophage factor inhibits adipocyte gene expression: an in vitro model of cachexia. Science. 1985 Aug 30;229(4716):867–869. [Abstract] [Google Scholar]
- Collins T, Lapierre LA, Fiers W, Strominger JL, Pober JS. Recombinant human tumor necrosis factor increases mRNA levels and surface expression of HLA-A,B antigens in vascular endothelial cells and dermal fibroblasts in vitro. Proc Natl Acad Sci U S A. 1986 Jan;83(2):446–450. [Europe PMC free article] [Abstract] [Google Scholar]
- Pober JS, Bevilacqua MP, Mendrick DL, Lapierre LA, Fiers W, Gimbrone MA., Jr Two distinct monokines, interleukin 1 and tumor necrosis factor, each independently induce biosynthesis and transient expression of the same antigen on the surface of cultured human vascular endothelial cells. J Immunol. 1986 Mar 1;136(5):1680–1687. [Abstract] [Google Scholar]
- Vilcek J, Palombella VJ, Henriksen-DeStefano D, Swenson C, Feinman R, Hirai M, Tsujimoto M. Fibroblast growth enhancing activity of tumor necrosis factor and its relationship to other polypeptide growth factors. J Exp Med. 1986 Mar 1;163(3):632–643. [Europe PMC free article] [Abstract] [Google Scholar]
- Dayer JM, Beutler B, Cerami A. Cachectin/tumor necrosis factor stimulates collagenase and prostaglandin E2 production by human synovial cells and dermal fibroblasts. J Exp Med. 1985 Dec 1;162(6):2163–2168. [Europe PMC free article] [Abstract] [Google Scholar]
- Nawroth PP, Bank I, Handley D, Cassimeris J, Chess L, Stern D. Tumor necrosis factor/cachectin interacts with endothelial cell receptors to induce release of interleukin 1. J Exp Med. 1986 Jun 1;163(6):1363–1375. [Europe PMC free article] [Abstract] [Google Scholar]
- Shacks SJ, Chiller J, Granger GA. Studies on in vitro models of cellular immunity: the role of T and B cells in the secretion of lymphotoxin. Cell Immunol. 1973 May;7(2):313–321. [Abstract] [Google Scholar]
- Leopardi E, Rosenau W. Production of alpha-lymphotoxin by human T-cell subsets. Cell Immunol. 1984 Jan;83(1):73–82. [Abstract] [Google Scholar]
- Schmid DS, Tite JP, Ruddle NH. DNA fragmentation: manifestation of target cell destruction mediated by cytotoxic T-cell lines, lymphotoxin-secreting helper T-cell clones, and cell-free lymphotoxin-containing supernatant. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1881–1885. [Europe PMC free article] [Abstract] [Google Scholar]
- Duke RC, Chervenak R, Cohen JJ. Endogenous endonuclease-induced DNA fragmentation: an early event in cell-mediated cytolysis. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6361–6365. [Europe PMC free article] [Abstract] [Google Scholar]
- Svedersky LP, Nedwin GE, Goeddel DV, Palladino MA., Jr Interferon-gamma enhances induction of lymphotoxin in recombinant interleukin 2-stimulated peripheral blood mononuclear cells. J Immunol. 1985 Mar;134(3):1604–1608. [Abstract] [Google Scholar]
- Gray PW, Aggarwal BB, Benton CV, Bringman TS, Henzel WJ, Jarrett JA, Leung DW, Moffat B, Ng P, Svedersky LP, et al. Cloning and expression of cDNA for human lymphotoxin, a lymphokine with tumour necrosis activity. Nature. 1984 Dec 20;312(5996):721–724. [Abstract] [Google Scholar]
- Lee SH, Aggarwal BB, Rinderknecht E, Assisi F, Chiu H. The synergistic anti-proliferative effect of gamma-interferon and human lymphotoxin. J Immunol. 1984 Sep;133(3):1083–1086. [Abstract] [Google Scholar]
- Stone-Wolff DS, Yip YK, Kelker HC, Le J, Henriksen-Destefano D, Rubin BY, Rinderknecht E, Aggarwal BB, Vilcek J. Interrelationships of human interferon-gamma with lymphotoxin and monocyte cytotoxin. J Exp Med. 1984 Mar 1;159(3):828–843. [Europe PMC free article] [Abstract] [Google Scholar]
- Ruggiero V, Tavernier J, Fiers W, Baglioni C. Induction of the synthesis of tumor necrosis factor receptors by interferon-gamma. J Immunol. 1986 Apr 1;136(7):2445–2450. [Abstract] [Google Scholar]
- Tsujimoto M, Yip YK, Vilcek J. Interferon-gamma enhances expression of cellular receptors for tumor necrosis factor. J Immunol. 1986 Apr 1;136(7):2441–2444. [Abstract] [Google Scholar]
- Pennica D, Nedwin GE, Hayflick JS, Seeburg PH, Derynck R, Palladino MA, Kohr WJ, Aggarwal BB, Goeddel DV. Human tumour necrosis factor: precursor structure, expression and homology to lymphotoxin. Nature. 1984 Dec 20;312(5996):724–729. [Abstract] [Google Scholar]
- Shirai T, Yamaguchi H, Ito H, Todd CW, Wallace RB. Cloning and expression in Escherichia coli of the gene for human tumour necrosis factor. Nature. 313(6005):803–806. [Abstract] [Google Scholar]
- Marmenout A, Fransen L, Tavernier J, Van der Heyden J, Tizard R, Kawashima E, Shaw A, Johnson MJ, Semon D, Müller R, et al. Molecular cloning and expression of human tumor necrosis factor and comparison with mouse tumor necrosis factor. Eur J Biochem. 1985 Nov 4;152(3):515–522. [Abstract] [Google Scholar]
- Nedwin GE, Naylor SL, Sakaguchi AY, Smith D, Jarrett-Nedwin J, Pennica D, Goeddel DV, Gray PW. Human lymphotoxin and tumor necrosis factor genes: structure, homology and chromosomal localization. Nucleic Acids Res. 1985 Sep 11;13(17):6361–6373. [Europe PMC free article] [Abstract] [Google Scholar]
- Nedospasov SA, Shakhov AN, Turetskaia RL, Mett VA, Georgiev GP. Molekuliarnoe klonirovanie genov cheloveka, kodiruiushchikh faktory nekroza opukholei: tandemnoe raspolozhenie al'fa- i beta-genov v korotkom segmente (6 tys. par nukleotidov) genoma cheloveka. Dokl Akad Nauk SSSR. 1985;285(6):1487–1490. [Abstract] [Google Scholar]
- Fransen L, Müller R, Marmenout A, Tavernier J, Van der Heyden J, Kawashima E, Chollet A, Tizard R, Van Heuverswyn H, Van Vliet A, et al. Molecular cloning of mouse tumour necrosis factor cDNA and its eukaryotic expression. Nucleic Acids Res. 1985 Jun 25;13(12):4417–4429. [Europe PMC free article] [Abstract] [Google Scholar]
- Pennica D, Hayflick JS, Bringman TS, Palladino MA, Goeddel DV. Cloning and expression in Escherichia coli of the cDNA for murine tumor necrosis factor. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6060–6064. [Europe PMC free article] [Abstract] [Google Scholar]
- Ito H, Yamamoto S, Kuroda S, Sakamoto H, Kajihara J, Kiyota T, Hayashi H, Kato M, Seko M. Molecular cloning and expression in Escherichia coli of the cDNA coding for rabbit tumor necrosis factor. DNA. 1986 Apr;5(2):149–156. [Abstract] [Google Scholar]
- Ito H, Shirai T, Yamamoto S, Akira M, Kawahara S, Todd CW, Wallace RB. Molecular cloning of the gene encoding rabbit tumor necrosis factor. DNA. 1986 Apr;5(2):157–165. [Abstract] [Google Scholar]
- Aggarwal BB, Kohr WJ, Hass PE, Moffat B, Spencer SA, Henzel WJ, Bringman TS, Nedwin GE, Goeddel DV, Harkins RN. Human tumor necrosis factor. Production, purification, and characterization. J Biol Chem. 1985 Feb 25;260(4):2345–2354. [Abstract] [Google Scholar]
- Aggarwal BB, Henzel WJ, Moffat B, Kohr WJ, Harkins RN. Primary structure of human lymphotoxin derived from 1788 lymphoblastoid cell line. J Biol Chem. 1985 Feb 25;260(4):2334–2344. [Abstract] [Google Scholar]
- Mori L, Lecoq AF, Robbiati F, Barbanti E, Righi M, Sinigaglia F, Clementi F, Ricciardi-Castagnoli P. Rearrangement and expression of the antigen receptor alpha, beta and gamma genes in suppressor antigen-specific T cell lines. EMBO J. 1985 Aug;4(8):2025–2030. [Europe PMC free article] [Abstract] [Google Scholar]
- Frischauf AM, Lehrach H, Poustka A, Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. [Abstract] [Google Scholar]
- McMaster GK, Beard P, Engers HD, Hirt B. Characterization of an immunosuppressive parvovirus related to the minute virus of mice. J Virol. 1981 Apr;38(1):317–326. [Europe PMC free article] [Abstract] [Google Scholar]
- Barnes WM, Bevan M, Son PH. Kilo-sequencing: creation of an ordered nest of asymmetric deletions across a large target sequence carried on phage M13. Methods Enzymol. 1983;101:98–122. [Abstract] [Google Scholar]
- Sahli R, McMaster GK, Hirt B. DNA sequence comparison between two tissue-specific variants of the autonomous parvovirus, minute virus of mice. Nucleic Acids Res. 1985 May 24;13(10):3617–3633. [Europe PMC free article] [Abstract] [Google Scholar]
- Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. [Europe PMC free article] [Abstract] [Google Scholar]
- Accolla RS, Scarpellino L, Carra G, Guardiola J. Trans-acting element(s) operating across species barriers positively regulate expression of major histocompatibility complex class II genes. J Exp Med. 1985 Oct 1;162(4):1117–1133. [Europe PMC free article] [Abstract] [Google Scholar]
- Accolla RS, Jotterand-Bellomo M, Scarpellino L, Maffei A, Carra G, Guardiola J. aIr-1, a newly found locus on mouse chromosome 16 encoding a trans-acting activator factor for MHC class II gene expression. J Exp Med. 1986 Jul 1;164(1):369–374. [Europe PMC free article] [Abstract] [Google Scholar]
- Church GM, Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. [Europe PMC free article] [Abstract] [Google Scholar]
- Butler ET, Chamberlin MJ. Bacteriophage SP6-specific RNA polymerase. I. Isolation and characterization of the enzyme. J Biol Chem. 1982 May 25;257(10):5772–5778. [Abstract] [Google Scholar]
- Weiss E, Golden L, Zakut R, Mellor A, Fahrner K, Kvist S, Flavell RA. The DNA sequence of the H-2kb gene: evidence for gene conversion as a mechanism for the generation of polymorphism in histocompatibilty antigens. EMBO J. 1983;2(3):453–462. [Europe PMC free article] [Abstract] [Google Scholar]
- Pustell J, Kafatos FC. A convenient and adaptable package of computer programs for DNA and protein sequence management, analysis and homology determination. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):643–655. [Europe PMC free article] [Abstract] [Google Scholar]
- Georgiev GP, Ilyin YV, Chmeliauskaite VG, Ryskov AP, Kramerov DA, Skryabin KG, Krayev AS, Lukanidin EM, Grigoryan MS. Mobile dispersed genetic elements and other middle repetitive DNA sequences in the genomes of Drosophila and mouse: transcription and biological significance. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 2):641–654. [Abstract] [Google Scholar]
- Beutler B, Krochin N, Milsark IW, Luedke C, Cerami A. Control of cachectin (tumor necrosis factor) synthesis: mechanisms of endotoxin resistance. Science. 1986 May 23;232(4753):977–980. [Abstract] [Google Scholar]
Associated Data
Articles from Nucleic Acids Research are provided here courtesy of Oxford University Press
Full text links
Read article at publisher's site: https://doi.org/10.1093/nar/14.19.7713
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC311791
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Mediators and Biomarkers of Inflammation in Meningitis: Cytokine and Peptidome Profiling of Cerebrospinal Fluid.
Biochemistry (Mosc), 81(11):1293-1302, 01 Nov 2016
Cited by: 11 articles | PMID: 27914455
The role of lymphotoxin signaling in the development of autoimmune pancreatitis and associated secondary extra-pancreatic pathologies.
Cytokine Growth Factor Rev, 25(2):125-137, 10 Jan 2014
Cited by: 8 articles | PMID: 24508087
Review
Epigenetic control of cytokine gene expression: regulation of the TNF/LT locus and T helper cell differentiation.
Adv Immunol, 118:37-128, 01 Jan 2013
Cited by: 39 articles | PMID: 23683942 | PMCID: PMC4118600
Review Free full text in Europe PMC
The unexpected role of lymphotoxin beta receptor signaling in carcinogenesis: from lymphoid tissue formation to liver and prostate cancer development.
Oncogene, 29(36):5006-5018, 05 Jul 2010
Cited by: 74 articles | PMID: 20603617
Review
Activation-dependent intrachromosomal interactions formed by the TNF gene promoter and two distal enhancers.
Proc Natl Acad Sci U S A, 104(43):16850-16855, 16 Oct 2007
Cited by: 54 articles | PMID: 17940009 | PMCID: PMC2040403
Go to all (56) article citations
Other citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Tumour necrosis factor and lymphotoxin genes map close to H-2D in the mouse major histocompatibility complex.
Nature, 325(6101):265-267, 01 Jan 1987
Cited by: 136 articles | PMID: 3027565
DNA sequence polymorphism at the human tumor necrosis factor (TNF) locus. Numerous TNF/lymphotoxin alleles tagged by two closely linked microsatellites in the upstream region of the lymphotoxin (TNF-beta) gene.
J Immunol, 147(3):1053-1059, 01 Aug 1991
Cited by: 117 articles | PMID: 1861069
[Cloning and structural analysis of genes coding for tumor necrosis factor and lymphotoxin in rabbits].
Mol Biol (Mosk), 23(6):1743-1750, 01 Nov 1989
Cited by: 1 article | PMID: 2633043
Porcine TNF: a review.
Vet Immunol Immunopathol, 47(3-4):187-201, 01 Aug 1995
Cited by: 12 articles | PMID: 8571540
Review