Abstract
Free full text

Toll-like receptor 1 as a possible target in non-alcoholic fatty liver disease
Abstract
Toll-like receptors (TLRs) in the liver compartment have repeatedly been attributed to the development of non-alcoholic fatty liver disease (NAFLD). Knowledge on TLR expression in blood cells and their relation to intestinal microbiota and NAFLD development is limited. Here, we determined TLR expression patterns in peripheral blood mononuclear cells (PBMCs) of NAFLD patients and controls, their relation to intestinal microbiota and the impact of TLRs found altered in NAFLD development. Markers of intestinal permeability in blood and TLR mRNA expression in PBMCs were determined in 37 NAFLD patients and 15 age-matched healthy controls. Fecal microbiota composition was evaluated in 21 NAFLD patients and 9 controls using 16S rRNA gene amplicon sequencing. Furthermore, TLR1−/− and C57BL/6 mice (n =
= 5–6/group) were pair-fed a liquid control or a fat-, fructose- and cholesterol-rich diet. Intestinal microbiota composition and markers of intestinal permeability like zonulin and bacterial endotoxin differed significantly between groups with the latter markers being significantly higher in NAFLD patients. Expression of TLR1-8 and 10 mRNA was detectable in PBMCs; however, only TLR1 expression, being higher in NAFLD patients, were significantly positively correlated with the prevalence of Holdemanella genus while negative correlations were found with Gemmiger and Ruminococcus genera. TLR1−/− mice were significantly protected from the development of diet-induced NAFLD when compared to wild-type mice. While intestinal microbiota composition and permeability differed significantly between NAFLD patients and healthy subjects, in PBMCs, only TLR1 expression differed between groups. Still, targeting these alterations might be a beneficial approach in the treatment of NAFLD in some patients.
5–6/group) were pair-fed a liquid control or a fat-, fructose- and cholesterol-rich diet. Intestinal microbiota composition and markers of intestinal permeability like zonulin and bacterial endotoxin differed significantly between groups with the latter markers being significantly higher in NAFLD patients. Expression of TLR1-8 and 10 mRNA was detectable in PBMCs; however, only TLR1 expression, being higher in NAFLD patients, were significantly positively correlated with the prevalence of Holdemanella genus while negative correlations were found with Gemmiger and Ruminococcus genera. TLR1−/− mice were significantly protected from the development of diet-induced NAFLD when compared to wild-type mice. While intestinal microbiota composition and permeability differed significantly between NAFLD patients and healthy subjects, in PBMCs, only TLR1 expression differed between groups. Still, targeting these alterations might be a beneficial approach in the treatment of NAFLD in some patients.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is by now one of the most common liver diseases worldwide affecting ~
~ 25% of the global general population with prevalence still increasing1,2. Accordingly, the demand for therapeutic interventions to treat patients at risk for the development of end-stage liver disease is tremendous and many have failed (for overview see3). As NAFLD is a disease slowly progressing along a spectrum ranging from hepatic steatosis defined as non-alcoholic fatty liver to inflammatory non-alcoholic steatohepatitis (NASH) and finally end-stage liver disease including cirrhosis and hepatocellular carcinoma4, opportunity to provide care to these patients seems to be large. However, as molecular mechanisms involved in the onset as well as the progression of the disease are still not fully understood, universally accepted strategies for prevention and therapy still mainly focus on life-style interventions, being often afflicted with low compliance and high relapse rates while drug-based therapies are still limited.
25% of the global general population with prevalence still increasing1,2. Accordingly, the demand for therapeutic interventions to treat patients at risk for the development of end-stage liver disease is tremendous and many have failed (for overview see3). As NAFLD is a disease slowly progressing along a spectrum ranging from hepatic steatosis defined as non-alcoholic fatty liver to inflammatory non-alcoholic steatohepatitis (NASH) and finally end-stage liver disease including cirrhosis and hepatocellular carcinoma4, opportunity to provide care to these patients seems to be large. However, as molecular mechanisms involved in the onset as well as the progression of the disease are still not fully understood, universally accepted strategies for prevention and therapy still mainly focus on life-style interventions, being often afflicted with low compliance and high relapse rates while drug-based therapies are still limited.
Toll-like receptors (TLRs) are evolutionarily conserved type I glycoprotein transmembrane receptors that posse a leucine-rich repeat in their ectodomains to recognize pathogen-associated molecular patterns (PAMP)5. Upon PAMP binding, conformation is changed leading to the formation of homo- or heterodimers, and subsequently, actions of cell signaling either involving an activation of myeloid differentiation primary response 88 (Myd88)- or TIR-domain-containing adapter-inducing interferon β (TRIF)/TRIF-related adaptor molecule (TRAM)-dependent signaling cascades6. TLRs are activated by a variety of bacterial, viral, fungal and parasitic antigens, but also endogenous ligands with each TLR responding to an unique subset of triggers7. In humans, 10 TLRs are expressed of which all have been detected in liver tissue8,9. Furthermore, studies in NAFLD patients and mice with diet-induced NAFLD suggest that expressions of TLRs in liver tissue are altered in settings of NAFLD being associated with intestinal barrier dysfunction and increased bacterial toxin levels9,10. Furthermore, many clinical studies indicate that patients with NAFLD exhibit a reduced bacterial diversity, sometimes also referred to as `gut dysbiosis´ (for overview see11). Indeed, studies suggest that an altered intestinal microbiota could lead to hepatic fat accumulation through several mechanisms including an increased translocation of bacterial toxins promoting low-grade inflammation and changes in gut permeability (for overview see12). These and other observations contribute to the concept of metabolic inflammation as an underlying modification that leads to the liver phenotype, but is also linked to extrahepatic morbidity in NAFLD (for overview see13).
However, whether similar changes in TLR expressions are also found in other tissues, like blood cells, and if these are related to alterations of intestinal microbiota composition and barrier function, and moreover, are indicative of disease targets have not been studied in-depth. Starting from this background, the first aim of the present study was to assess TLR expression patterns in peripheral blood mononuclear cells (PBMCs) derived from NAFLD patients and age-matched controls and their relation to intestinal microbiota composition and barrier function. In a second step, the impact of differences found regarding TLR1 expression between NAFLD patients and controls on disease development was assessed in a dietary mouse model employing TLR1−/− mice.
Results
Characteristics of NAFLD patients and controls
Age was similar between NAFLD patients and controls (Table (Table1),1), while gender differed by trend between groups (p =
= 0.0635). The mean value of steatosis stage of NAFLD patients was 1.7 and was based on the score. NAFLD patients suffered from a hepatic steatosis ranging from mild to moderate. Activity of transaminases and gamma glutamyl transferase (γ-GT) in plasma but also markers of glucose and lipid metabolism as well as levels of C-reactive protein (CRP), plasminogen activator inhibitor 1 (PAI-1), uric acid, leptin and body mass index (BMI) as well as waist circumference were significantly higher in NAFLD patients than in controls. Adiponectin levels were significantly lower in NAFLD patients than in controls. Leptin to adiponectin ratio, shown to be indicative of visceral adiposity14, was also significantly higher in NAFLD patients than in controls (Table 1). Furthermore, both, systolic and diastolic blood pressure were significantly higher in patients with NAFLD than in controls (Table (Table1)1) with 70% of the NAFLD patients suffering from hypertension. 14% of patients had diabetes type 2 (fasting blood glucose levels
0.0635). The mean value of steatosis stage of NAFLD patients was 1.7 and was based on the score. NAFLD patients suffered from a hepatic steatosis ranging from mild to moderate. Activity of transaminases and gamma glutamyl transferase (γ-GT) in plasma but also markers of glucose and lipid metabolism as well as levels of C-reactive protein (CRP), plasminogen activator inhibitor 1 (PAI-1), uric acid, leptin and body mass index (BMI) as well as waist circumference were significantly higher in NAFLD patients than in controls. Adiponectin levels were significantly lower in NAFLD patients than in controls. Leptin to adiponectin ratio, shown to be indicative of visceral adiposity14, was also significantly higher in NAFLD patients than in controls (Table 1). Furthermore, both, systolic and diastolic blood pressure were significantly higher in patients with NAFLD than in controls (Table (Table1)1) with 70% of the NAFLD patients suffering from hypertension. 14% of patients had diabetes type 2 (fasting blood glucose levels ≥
≥ 7.0 mmol/l) and 64% had a metabolic syndrome as defined by the WHO/International Diabetes Federation diagnostic criteria (WHO/IDF, 2006).
7.0 mmol/l) and 64% had a metabolic syndrome as defined by the WHO/International Diabetes Federation diagnostic criteria (WHO/IDF, 2006).
Table 1
Characteristics of study participants.
| C | NAFLD | |
|---|---|---|
| n | 15 | 37 |
| Sex (m/f) | 5/10 | 24/13 |
| Age | 46.2 ± ± 1.6 1.6 | 47.7 ± ± 2.2 2.2 |
| BMI (kg/m2) | 23.6 ± ± 0.7 0.7 | 31.6 ± ± 1.0* 1.0* |
| Waist circumference in cm (m/f) | 86 ± ± 2/73 2/73 ± ± 2 2 | 111 ± ± 3*/105 3*/105 ± ± 5* 5* |
| Steatosis (stage) | 0 ± ± 0 0 | 1.7 ± ± 0.1* 0.1* |
| ALT (U/l) | 19.1 ± ± 1.6 1.6 | 67.0 ± ± 7.0* 7.0* |
| AST (U/l) | 20.1 ± ± 2.1 2.1 | 41.0 ± ± 2.8* 2.8* |
| γ-GT (U/l) | 17.9 ± ± 2.2 2.2 | 102.9 ± ± 15.8* 15.8* |
| Systolic blood pressure (mmHg) | 125.5 ± ± 2.7 2.7 | 140.1 ± ± 3.2* 3.2* |
| Diastolic blood pressure (mmHg) | 80.9 ± ± 2.0 2.0 | 89.8 ± ± 1.6* 1.6* |
| Triglycerides (mg/dl) | 83.5 ± ± 15.4 15.4 | 160.9 ± ± 15.2* 15.2* |
| Total cholesterol (mg/dl) | 189.4 ± ± 9.1 9.1 | 219.8 ± ± 6.7* 6.7* |
| LDL (mg/dl) | 103.3 ± ± 7.4 7.4 | 136.5 ± ± 6.3* 6.3* |
| HDL in mg/dl (m/f) | 60.4 ± ± 4.3/76.9 4.3/76.9 ± ± 3.9 3.9 | 45.5 ± ± 2.9*/61.6 2.9*/61.6 ± ± 5.5* 5.5* |
| Insulin (µU/ml) | 4.2 ± ± 0.2 0.2 | 14.6 ± ± 1.9* 1.9* |
| Fasting glucose (mg/dl) | 80.3 ± ± 2.8 2.8 | 103.0 ± ± 4.3* 4.3* |
| HOMA-IR | 0.9 ± ± 0.1 0.1 | 4.2 ± ± 1.0* 1.0* |
| Leptin (ng/ml) | 4.4 ± ± 0.7 0.7 | 12.1 ± ± 1.6* 1.6* |
| Adiponectin (ng/ml) | 53.2 ± ± 6.8 6.8 | 24.0 ± ± 2.9* 2.9* |
| Leptin/adiponectin | 0.33 ± ± 0.2 0.2 | 0.74 ± ± 0.2* 0.2* |
| PAI-1 (U/l) | 10.6 ± ± 2.2 2.2 | 45.0 ± ± 3.8* 3.8* |
| CRP (mg/l) | 1.6 ± ± 0.5 0.5 | 5.0 ± ± 1.2* 1.2* |
| Uric acid (mg/dl) | 4.2 ± ± 0.3 0.3 | 6.2 ± ± 0.3* 0.3* |
Data are shown as total numbers and means ±
± SEM, respectively, n
SEM, respectively, n =
= 14–15 controls and n
14–15 controls and n =
= 32–37 NAFLD patients, *p
32–37 NAFLD patients, *p ≤
≤ 0.05 compared to controls.
0.05 compared to controls.
γ-GT gamma glutamyl transferase, ALT alanine aminotransferase, AST aspartate aminotransferase, BMI body mass index, C healthy controls, CRP C-reactive protein, NAFLD patients with non-alcoholic fatty liver disease, PAI-1 plasminogen activator inhibitor 1.
Fecal microbiota in patients with NAFLD and controls
While a total of 1.103 operational taxonomic units (OTUs) were shared between the NAFLD patients and controls, the number of unshared OTUs in the NAFLD patients was almost three times higher than the unshared OTUs in controls (Fig. 1A). However, most of the unshared OTUs were present in low abundances. The Principal Coordinates Analysis (PCoA) showed a clustering of microbial communities in NAFLD patients and the controls (Fig. 1B). The microbial community of both groups was statistically different when analysed by PERMONOVA (p =
= 0.009). Canonical correspondence analysis (CCA) and redundance analysis (RDA) tests indicated that NAFLD patients and controls have a unique composition (p
0.009). Canonical correspondence analysis (CCA) and redundance analysis (RDA) tests indicated that NAFLD patients and controls have a unique composition (p =
= 0.001 and p
0.001 and p =
= 0.003, respectively; Fig. 1C,D). The average similarity among replicates within the two groups was 37% in NAFLD patients and 36% in controls. The diversity of the bacterial communities measured by the Shannon’s index and species richness were statistically lower in NAFLD patients compared with controls (Fig. 1E,F). Species evenness and the Simpson’s index did not show statistical differences between the groups (Fig. 1G,H). The relative abundance of bacterial genera in feces of NAFLD patients and controls is shown in Fig. 2A. Relative abundances of Alistipes and Oscillibacter were significantly lower in feces obtained from patients with NAFLD than in controls (Fig. 2B,C). Relative abundances of Blautia, Fusicatenibacter, Dorea and Ruminococcus 2 were significantly higher in feces obtained from patients with NAFLD than in feces of controls (Fig. 2D–G, p
0.003, respectively; Fig. 1C,D). The average similarity among replicates within the two groups was 37% in NAFLD patients and 36% in controls. The diversity of the bacterial communities measured by the Shannon’s index and species richness were statistically lower in NAFLD patients compared with controls (Fig. 1E,F). Species evenness and the Simpson’s index did not show statistical differences between the groups (Fig. 1G,H). The relative abundance of bacterial genera in feces of NAFLD patients and controls is shown in Fig. 2A. Relative abundances of Alistipes and Oscillibacter were significantly lower in feces obtained from patients with NAFLD than in controls (Fig. 2B,C). Relative abundances of Blautia, Fusicatenibacter, Dorea and Ruminococcus 2 were significantly higher in feces obtained from patients with NAFLD than in feces of controls (Fig. 2D–G, p ≤
≤ 0.05 for all). The result of the Linear discriminant analysis coupled with Effect Size (LEfSe) showed that the genera Blautia, Fusicatenibacter, Dorea, Clostridium XIVa and an unclassified member of Succinivibrionaceae (Linear Discriminant Analysis score, LDA
0.05 for all). The result of the Linear discriminant analysis coupled with Effect Size (LEfSe) showed that the genera Blautia, Fusicatenibacter, Dorea, Clostridium XIVa and an unclassified member of Succinivibrionaceae (Linear Discriminant Analysis score, LDA >
> 2.5) were more abundant in NAFLD patients than in controls whereas nine other taxa were predominately found in controls (Figure S1). At species level, Blautia sp. and Collinsella aerofaciens were detected in higher abundance in feces of NAFLD patients in comparison to controls (Fig. 2H,I).
2.5) were more abundant in NAFLD patients than in controls whereas nine other taxa were predominately found in controls (Figure S1). At species level, Blautia sp. and Collinsella aerofaciens were detected in higher abundance in feces of NAFLD patients in comparison to controls (Fig. 2H,I).
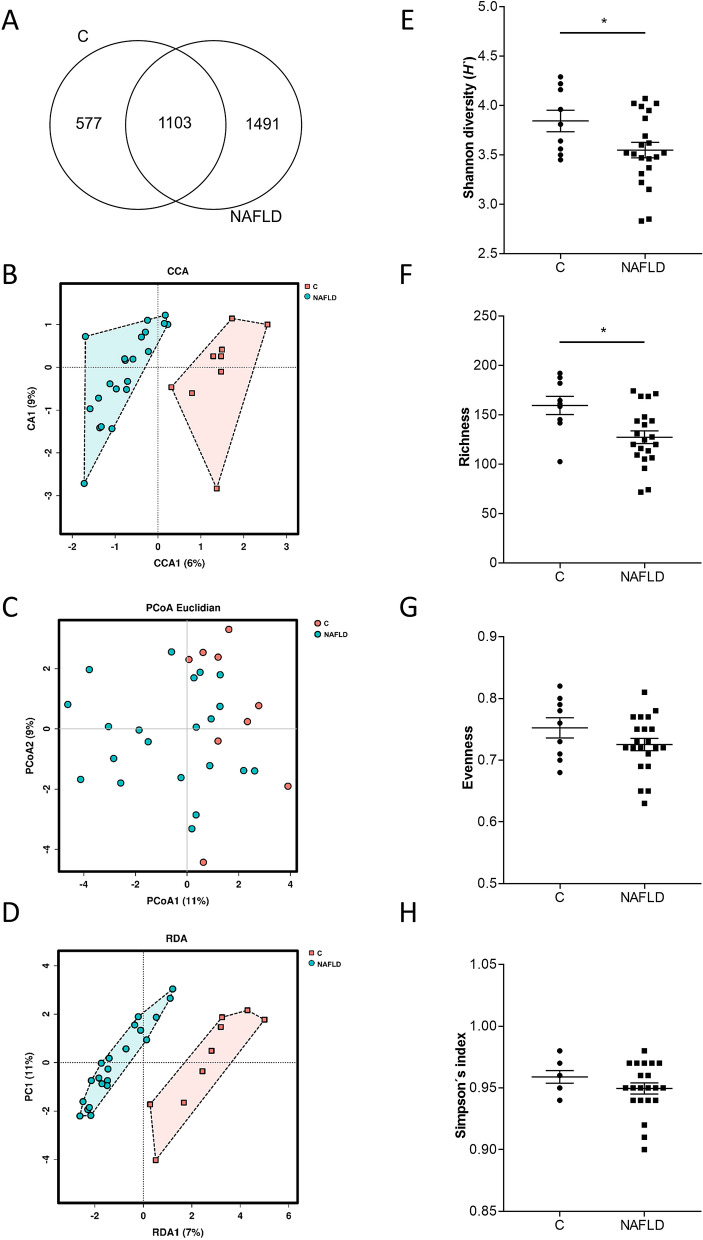
Global bacterial community structure and diversity indexes of intestinal microbiota in NAFLD patients and controls. (A) Venn diagram representing the operational taxonomic units (OTUs)/species of the unfiltered reads, (B) Principal Coordinates Analysis (PCoA) with Elucidian dissimilarity distance, (C) canonical correspondance analysis (CCA) and (D) redundance analysis (RDA), (E) Shannon diversity index, (F) species richness, (G) species evenness and (H) Simpon’s index. Each dot represents the microbial community of each proband. Data are shown as means ±
± SEM, n
SEM, n =
= 9 controls, n
9 controls, n =
= 21 NAFLD patients, *p
21 NAFLD patients, *p ≤
≤ 0.05. C healthy controls, NAFLD patients with non-alcoholic fatty liver disease.
0.05. C healthy controls, NAFLD patients with non-alcoholic fatty liver disease.
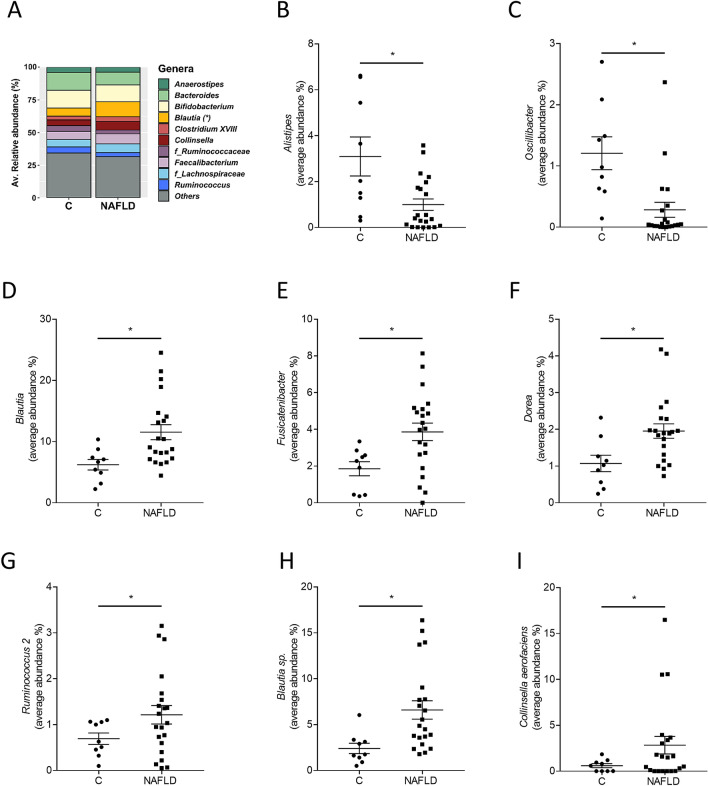
Microbial composition in feces of NAFLD patients and controls. (A) Relative abundance of bacterial genera in feces of NAFLD patients and controls as well as the average abundances of (B–G) genera and (H, I) species that are statistically different. Data are shown as means ±
± SEM, n
SEM, n =
= 9 controls, n
9 controls, n =
= 21 NAFLD patients, *p
21 NAFLD patients, *p ≤
≤ 0.05. C healthy controls, NAFLD patients with non-alcoholic fatty liver disease.
0.05. C healthy controls, NAFLD patients with non-alcoholic fatty liver disease.
Markers of intestinal permeability in NAFLD patients and controls in relation to TLR1 mRNA expression in PBMCs
Bacterial endotoxin levels in plasma and zonulin concentration in serum were significantly higher in patients with NAFLD than in controls (Fig. 3A,B). Citrulline concentrations trended to be numerically higher in NAFLD patients compared to controls (p =
= 0.07; Fig. 3C). In contrast, plasma levels of lipoteichoic acid (LTA), being a ligand of TLR2 and 6 as well as TLR115, were not different between controls and NAFLD patients (Fig. 3D).
0.07; Fig. 3C). In contrast, plasma levels of lipoteichoic acid (LTA), being a ligand of TLR2 and 6 as well as TLR115, were not different between controls and NAFLD patients (Fig. 3D).
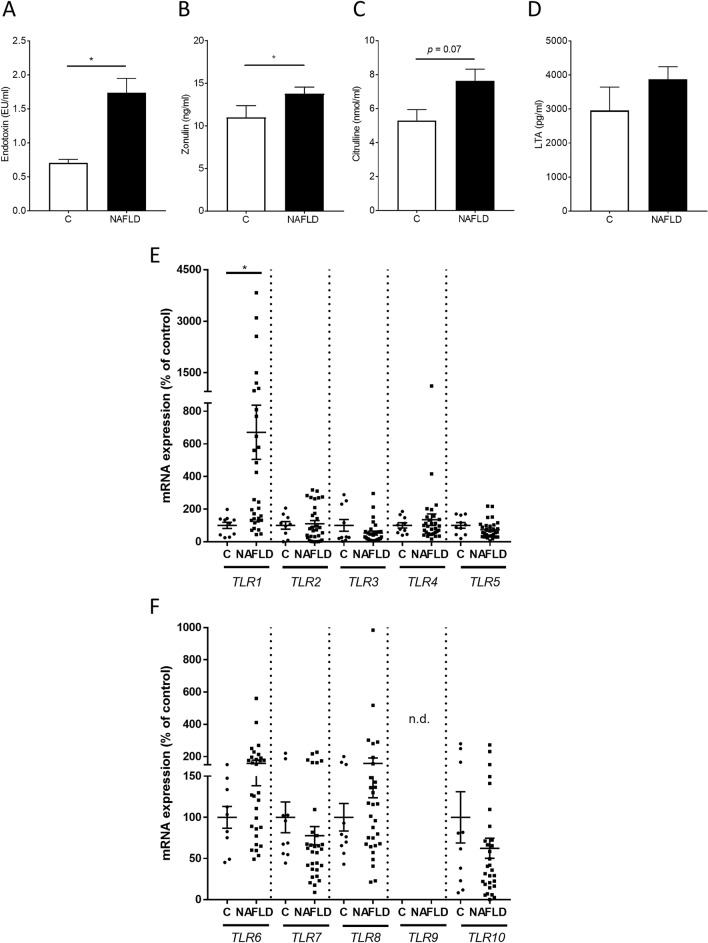
Markers of intestinal permeability and TLR expression in human PBMCs of NAFLD patients and controls. (A) Endotoxin, (B) zonulin, (C) citrulline and (D) lipoteichoic acid in plasma as well as (E) TLR1 to TLR5 and (F) TLR6 to TLR10 mRNA expression. Data are shown as means ±
± SEM, n
SEM, n =
= 10–14 controls, n
10–14 controls, n =
= 31–36 NAFLD patients, *p
31–36 NAFLD patients, *p ≤
≤ 0.05. C healthy controls, LTA lipoteichoic acid, NAFLD patients with non-alcoholic fatty liver disease, PBMCs peripheral blood mononuclear cells, TLR toll-like receptor.
0.05. C healthy controls, LTA lipoteichoic acid, NAFLD patients with non-alcoholic fatty liver disease, PBMCs peripheral blood mononuclear cells, TLR toll-like receptor.
Expression of TLRs and dependent signaling cascades in NAFLD patients and controls
Apart from TLR9 being under the level of detection, expression of TLR1-10 was detectable in human PBMCs isolated from both, patients with NAFLD and controls (Fig. 3E,F). However, only expression of TLR1 mRNA differed significantly between groups with TLR1 expression being significantly higher in PBMCs isolated from patients with NAFLD than in controls. Expression of the TLR adaptor protein MYD88 as well as expression of TIR domain containing adaptor protein (TIRAP) and interferon regulatory factor 3 (IRF3) were similar between groups (Table S1).
Correlation analysis of TLR1 expression in PBMCs with intestinal microbiota and markers of intestinal permeability
To determine if relative abundance of certain bacterial genera or strains detected in feces of patients with NAFLD and controls as well as markers of intestinal permeability were related to TLR1 mRNA expression in PBMCs, a correlation analysis was performed. The genus Holdemanella was significantly positive related to the mRNA expression of TLR1 in PBMCs. In contrast, for the genera Ruminococcus and Gemmiger, a significantly negative correlation with mRNA expression of TLR1 in PBMCs was found (Fig. 4A; Table S2). Levels of endotoxin in plasma were significantly positive correlated with TLR1 mRNA expression in PBMCs with a similar trend being also found for LTA plasma levels and TLR1 mRNA expression in PBMCs (Fig. 4B,C). However, no associations between further markers of intestinal integrity and the mRNA expression of TLR1 in PBMCs were found (data not shown).
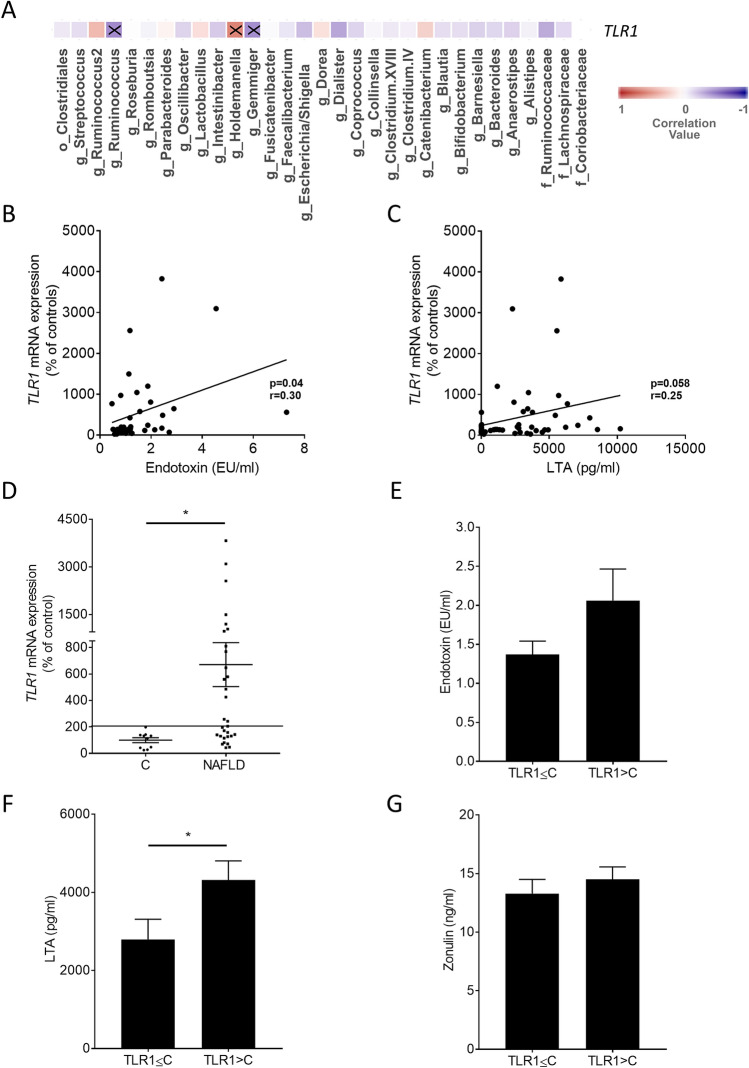
Correlation analyses of TLR1 mRNA expression and bacterial taxa or markers of intestinal permeability as well as markers of intestinal permeability stratified to TLR1. (A) Heatmap showing the Pearson correlation between the bacterial taxa and TLR1 and correlation analysis of TLR1 mRNA expression in human PBMCs with (B) endotoxin and (C) LTA concentrations in plasma. (D) TLR1 mRNA expression, concentration of (E) endotoxin, (F) LTA and (G) zonulin stratified according to TLR1. Letters before the name of bacterial communities represent the taxonomical level, g =
= genus, f
genus, f =
= family, o
family, o =
= order. Higher values in red above 0 show a positive correlation, while values lower than 0 in blue indicate a negative correlation. Correlations marked with an X are statistically significant. Line indicates the cut-off of 200% TLR1 mRNA expression. TLR1 expression
order. Higher values in red above 0 show a positive correlation, while values lower than 0 in blue indicate a negative correlation. Correlations marked with an X are statistically significant. Line indicates the cut-off of 200% TLR1 mRNA expression. TLR1 expression ≤
≤ C, patients with NAFLD showing a TLR1 mRNA expression in PBMCs
C, patients with NAFLD showing a TLR1 mRNA expression in PBMCs ≤
≤ TLR1 mRNA expression in PBMCs of C; TLR1 expression
TLR1 mRNA expression in PBMCs of C; TLR1 expression >
> C, patients with NAFLD showing a TLR1 mRNA expression in PBMCs
C, patients with NAFLD showing a TLR1 mRNA expression in PBMCs >
> TLR1 mRNA expression in PBMCs of C. Data are shown as means
TLR1 mRNA expression in PBMCs of C. Data are shown as means ±
± SEM, n
SEM, n =
= 7–14 controls, n
7–14 controls, n =
= 16–31 NAFLD patients, *p
16–31 NAFLD patients, *p ≤
≤ 0.05. C healthy controls, LTA lipoteichoic acid, NAFLD patients with non-alcoholic fatty liver disease, PBMCs peripheral blood mononuclear cells, TLR toll-like receptor.
0.05. C healthy controls, LTA lipoteichoic acid, NAFLD patients with non-alcoholic fatty liver disease, PBMCs peripheral blood mononuclear cells, TLR toll-like receptor.
When further analyzing mRNA expression of TLR1 in PBMCs of patients with NAFLD, it was found that TLR1 mRNA was clustered with 14 NAFLD patients showing TLR1 mRNA expression in PBMCs similar or below to that of controls and 17 NAFLD patients having TLR1 mRNA expression in PBMCs above that of controls (highest control: 198%, Fig. 4D). Interestingly, when clustering plasma endotoxin, LTA and zonulin levels accordingly, LTA plasma levels of NAFLD patients with a high TLR1 mRNA expression (> 198%) were found to be significantly higher than those of patients with low TLR1 mRNA expression in PBMCs (Fig. 4F). Differences alike were not found for endotoxin and zonulin levels (Fig. 4E,G). To determine if endotoxin and LTA, respectively, affect TLR1 expression in human PBMCs, PBMCs isolated from healthy young adults were challenged with 100 ng/ml lipopolysaccharide (LPS) or 10 µg/ml LTA for 24 and 48 h and expression of TLR1 was determined. While after 24 h, expression of TLR1 was unchanged in cells challenged with LPS or LTA, TLR1 mRNA expression was super-induced after 48 h (Fig. 5).
198%) were found to be significantly higher than those of patients with low TLR1 mRNA expression in PBMCs (Fig. 4F). Differences alike were not found for endotoxin and zonulin levels (Fig. 4E,G). To determine if endotoxin and LTA, respectively, affect TLR1 expression in human PBMCs, PBMCs isolated from healthy young adults were challenged with 100 ng/ml lipopolysaccharide (LPS) or 10 µg/ml LTA for 24 and 48 h and expression of TLR1 was determined. While after 24 h, expression of TLR1 was unchanged in cells challenged with LPS or LTA, TLR1 mRNA expression was super-induced after 48 h (Fig. 5).
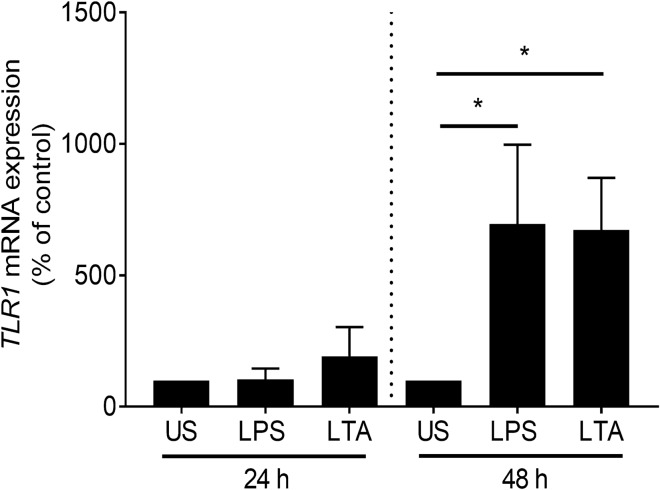
TLR1 mRNA expression in human PBMCs in healthy probands. Human PBMCs were stimulated ex vivo with 100 ng/ml LPS or 10 µg/ml LTA for 24 or 48 h. Data are shown as means ±
± SEM, n
SEM, n =
= 6, *p
6, *p ≤
≤ 0.05. LPS lipopolysaccharide, LTA lipoteichoic acid, PBMCs peripheral blood mononuclear cells, TLR toll-like receptor, US unstimulated.
0.05. LPS lipopolysaccharide, LTA lipoteichoic acid, PBMCs peripheral blood mononuclear cells, TLR toll-like receptor, US unstimulated.
Expression of Tlrs in PBMCs and of hepatic Tlr1 in mice with diet-induced NAFLD as well as plasma levels of LTA and endotoxin in portal vein
To determine if changes in Tlr1 mRNA expression in PBMCs are related to changes in expression of Tlr1 in liver, mice were either pair-fed a fat-, fructose- and cholesterol-rich diet (FFC) for six weeks to induce NAFLD or a control diet (C). As the yield of PBMCs and mRNA was rather low, samples of two mice were pooled so that a total of four samples obtained from eight mice per group were analyzed. Accordingly, no statistical analysis was performed. In line with the findings in humans, Tlr1 expression was also markedly higher in PBMCs isolated from mice with diet-induced NAFLD than in C-fed mice (Fig. 6A). Furthermore, Tlr1 mRNA expression in liver tissue was also significantly higher in mice with NAFLD than in controls (Fig. 6B). Also, in line with the findings in humans, concentrations of bacterial endotoxin in plasma obtained from the portal vein of mice with NAFLD were also significantly higher than in control animals while LTA levels, varying considerably between animals, did not differ (Fig. 6C,D).
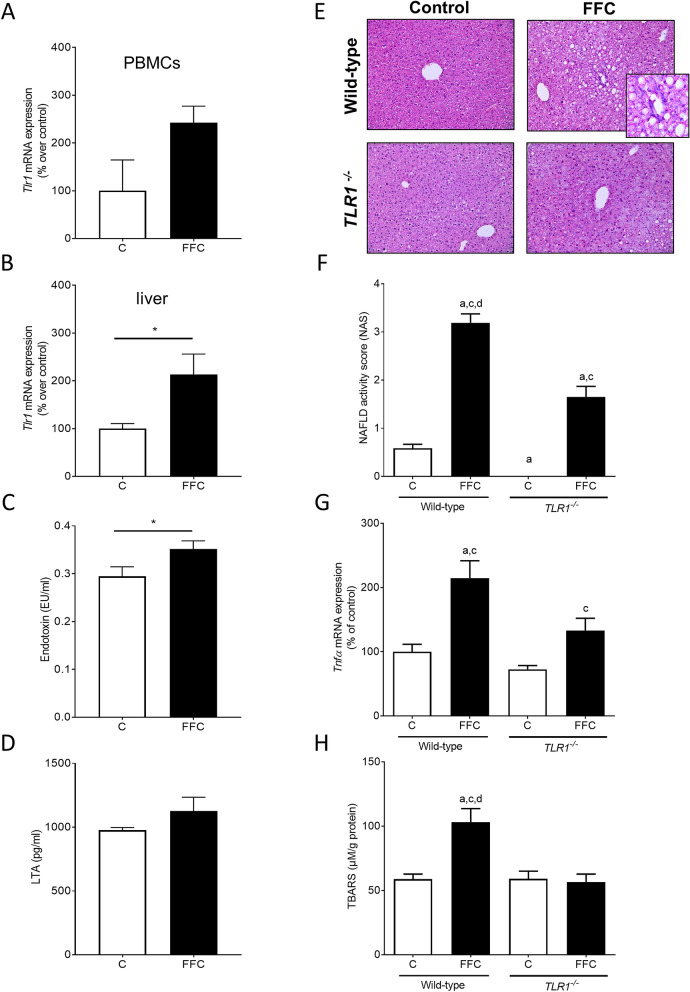
Effect of TLR1 on markers of intestinal permeability and indices of liver damage in FFC-fed mice. Tlr1 mRNA expression in (A) PBMCs and (B) liver tissue, (C) endotoxin concentration as well as (D) LTA levels in plasma of C- and FFC-fed wild-type mice. (E) Representative pictures (magnification ×200 and ×400) of hematoxylin and eosin staining and (F) NAFLD activity score (NAS) in liver tissue, (G) mRNA expression of Tnfα in liver tissue and (H) concentration of TBARS in liver tissue of C- and FFC-fed TLR1−/− and wild-type mice. Data are shown as means ±
± SEM, n
SEM, n =
= 5–8, except of (A) as yield of PBMCs was rather low, 2 samples were pooled respectively and for (D): n
5–8, except of (A) as yield of PBMCs was rather low, 2 samples were pooled respectively and for (D): n =
= 4 in C-fed mice, *p
4 in C-fed mice, *p ≤
≤ 0.05 between C- and FFC-fed wild-type mice; ap
0.05 between C- and FFC-fed wild-type mice; ap ≤
≤ 0.05 compared to C-fed wild-type mice; cp
0.05 compared to C-fed wild-type mice; cp ≤
≤ 0.05 compared to C-fed TLR1−/− mice; dp
0.05 compared to C-fed TLR1−/− mice; dp ≤
≤ 0.05 compared to FFC-fed TLR1−/− mice. C control diet, FFC fat-, fructose- and cholesterol-rich diet, LTA lipoteichoic acid, PBMCs peripheral blood mononuclear cells, TBARS thiobarbituric acid reactive substances, Tlr toll-like receptor, Tnf tumor necrosis factor.
0.05 compared to FFC-fed TLR1−/− mice. C control diet, FFC fat-, fructose- and cholesterol-rich diet, LTA lipoteichoic acid, PBMCs peripheral blood mononuclear cells, TBARS thiobarbituric acid reactive substances, Tlr toll-like receptor, Tnf tumor necrosis factor.
Liver histology, clinical parameters and markers of inflammation as well as lipid peroxidation in wild-type mice and TLR1−/− mice fed a diet to induce NAFLD
To determine if TLR1 is critical in the development of NAFLD, TLR1−/− mice and wild-type mice were fed a FFC diet for eight weeks. Liver weight and liver to body weight ratio were significantly higher in FFC-fed TLR1−/− and wild-type mice than in C-fed groups (Table (Table2).2). Development of steatosis and even more of early signs of inflammation were markedly attenuated in FFC-fed TLR1−/− when compared to FFC-fed wild-type animals. Indeed, NAFLD activity score (NAS) was significantly lower in TLR1−/− mice fed the FFC diet when compared to FFC-fed wild-type mice (Fig. 6E,F). However, NAS in FFC-fed TLR1−/− mice was still significantly higher than in both control groups. Expressions of F4/80 and tumor necrosis factor alpha (Tnfα) mRNA were also significantly higher in livers of FFC-fed wild-type mice when compared to both control groups while in TLR1−/− mice fed the FFC, expression was only significantly higher than in C-fed TLR1−/− mice (Table (Table2;2; Fig. 6G). In line with these findings, concentration of thiobarbituric acid reactive substances (TBARS) in liver tissue was only significantly higher in FFC-fed wild-type mice than in both C-fed mice. Concentration of TBARS in livers of TLR1−/− mice fed the FFC was at the level of controls (Fig. 6H). Neither alanine aminotransferase (ALT) nor aspartate aminotransferase (AST) or fasting glucose levels differed between groups irrespective of diet fed (Table 2).
Table 2
Effect of deletion of TLR1 on caloric intake, body- and liver weight and liver damage in FFC-fed mice.
| Wild-type | TLR1−/− | |||
|---|---|---|---|---|
| C | FFC | C | FFC | |
| Caloric intake (kcal/g bw) | 0.45 ± ± 0.0 0.0 | 0.44 ± ± 0.0 0.0 | 0.42 ± ± 0.0 0.0 | 0.42 ± ± 0.0 0.0 |
| Body weight (g) | 22.1 ± ± 0.4 0.4 | 22.7 ± ± 0.6 0.6 | 22.5 ± ± 0.8 0.8 | 23.7 ± ± 0.6 0.6 |
| Liver weight (g) | 1.2 ± ± 0.1 0.1 | 1.6 ± ± 0.1a,c 0.1a,c | 1.1 ± ± 0.1 0.1 | 1.5 ± ± 0.1a,c 0.1a,c |
| Liver:body weight ratio (%) | 5.3 ± ± 0.2 0.2 | 6.9 ± ± 0.2a,c 0.2a,c | 4.9 ± ± 0.2 0.2 | 6.5 ± ± 0.2a,c 0.2a,c |
| ALT (U/l) | 15.7 ± ± 2.6 2.6 | 16.1 ± ± 1.2 1.2 | 16.5 ± ± 3.1 3.1 | 20.2 ± ± 1.5 1.5 |
| AST (U/l) | 40.8 ± ± 1.2 1.2 | 43.7 ± ± 2.4 2.4 | 44.6 ± ± 3.0 3.0 | 47.9 ± ± 0.8 0.8 |
| Fasted glucose concentration (mg/dl) | 102.0 ± ± 3.6 3.6 | 92.0 ± ± 6.3 6.3 | 81.0 ± ± 4.0 4.0 | 87.8 ± ± 7.9 7.9 |
| F4/80 mRNA expression (% of control) | 100 ± ± 6.0 6.0 | 192.1 ± ± 18.5a,c 18.5a,c | 112.1 ± ± 77.8 77.8 | 158.4 ± ± 17.8a 17.8a |
Data are shown as means ±
± SEM, n
SEM, n =
= 5–6, ap
5–6, ap ≤
≤ 0.05 compared to C-fed wild-type mice, cp
0.05 compared to C-fed wild-type mice, cp ≤
≤ 0.05 compared to C-fed TLR1−/− mice.
0.05 compared to C-fed TLR1−/− mice.
ALT alanine aminotransferase, AST aspartate aminotransferase, C control diet, FFC fat-, fructose-, and cholesterol-rich diet, TLR toll-like receptor.
Discussion
NAFLD is by now the leading liver disease in the world16. And while results of epidemiological studies suggest that overweight and insulin resistance are the key risk factors for the development of NAFLD, results of clinical and experimental studies also suggest that changes of intestinal microbiota composition and intestinal barrier function may contribute to disease onset and progression (for overview see17). In line with the findings of others18, results of the present study suggest that NAFLD is associated with marked changes in intestinal microbiota composition and lower diversity of microbial communities. Indeed, the lower relative abundance of Alistipes and Oscillibacter species in NAFLD patients in comparison to controls is in line with the findings of others showing a reduction of Alistipes in patients with liver fibrosis and cirrhosis and of Oscillibacter in NAFLD patients19–21 (and for overview see22). Furthermore, a higher relative abundance of Blautia, Fusicatenibacter and Dorea as well as Ruminococcus 2 in NAFLD patients in comparison to healthy controls was reported by others studying humans with this liver disease19,23. However, others reported that NAFLD was associated with a decrease in the relative abundance of Blautia and Ruminococcus24. Further studies are needed to determine the physiological impact of these alterations, and herein, especially the impact of changes in the abundance of specific bacteria on intestinal barrier function but also the liver.
The changes found in intestinal microbiota abundance and composition were associated with higher levels of zonulin protein, being proposed to be indicative of intestinal barrier function and integrity25. In line with these findings, bacterial endotoxin levels in plasma of NAFLD patients were also significantly higher, all suggesting that intestinal barrier function of NAFLD patients was impaired, and subsequently, translocation of bacterial toxins was increased. These findings are in line with the findings of others and earlier studies of our own group in adults and children with early and progressed signs of the disease all suggesting that the development and progression of NAFLD is associated with changes in intestinal barrier function and an increased translocation of bacterial endotoxin26–29. Somewhat contrasting the findings for bacterial endotoxin and the other markers of intestinal barrier function, LTA levels in plasma were not markedly higher in NAFLD patients; however, we found that data varied considerably and were sort of clustered. Indeed, when delineating LTA levels according to TLR1 expression in PBMCs, we found that those patients with a TLR1 expression higher than the highest expression found in healthy controls also had significantly higher LTA levels in their blood than those with an expression at the level of controls. In line with these findings, in mice with a FFC-induced NAFLD, Tlr1 mRNA expression in PBMCs was also markedly higher going also along with significantly higher bacterial endotoxin levels while LTA levels, varying considerably, were similar to controls. It might be that in mice, duration of feeding was not yet sufficient to stably increase LTA levels in plasma, yet. Furthermore, it has been shown before that similar to TLR4, surface resident TLR1 forming heterodimers with TLR2 or TLR6, clears its ligands through forming endosomes30. It could very well be, that in FFC-fed mice displaying only early signs of steatosis with beginning inflammatory alterations, clearance of LTA through TLR1 in intestinal tissue and liver was efficient enough to avoid a marked increase of LTA in the portal vein. Indeed, Tlr1 mRNA expression was significantly higher in livers of FFC-fed mice when compared to control animals. Interestingly, in human samples, similar relations were not found for TLR2 or TLR6, both shown to form heterodimers with TLR1 and to be critical for the responsiveness of TLR1 to bacterial toxins31. However, while expressions of TLR2 and TLR6 were not elevated in PBMCs of patients with NAFLD, expression of both receptors was still detectable. Further studies are needed to determine the interplay of TLR1, TLR2 and TLR6 in PBMCs in NAFLD patients. Somewhat supporting the hypothesis that TLR1 expression is directly related to plasma levels of bacterial toxins, and herein, especially LTA and endotoxin, expression of TLR1 mRNA was found to be significantly induced upon stimulation of both, LTA and LPS. Furthermore, expression of TLR1 in PBMCs of NAFLD patients and controls was positively related to plasma levels of bacterial endotoxin. It has been shown by others before, that number of TLR1+ monocytes is increased upon stimulation with LTA32. Furthermore, it has been shown that TLR1 is expressed in all leukocytes33 and that in the present of TLR2 may also be activated by certain LPS preparations31.
Interestingly and contrasting the findings of us and others assessing expression of TLRs in liver tissue of both rodents and humans with NAFLD9,10 but also studies in PBMCs isolated from patients with chronic hepatitis B and chronic hepatitis B-related liver failure34, only TLR1 expression was found to be higher in PBMCs of NAFLD patients when compared to healthy controls. Differences between the studies of Wang et al. and our study could have resulted from differences in disease etiology, e.g. virus infection vs. NAFLD. It could also be that the difference between the findings in liver and blood cells are related to the fact that PBMCs are a mixed immune cell population35. Indeed, it has been shown before that while TLRs are traditionally associated with cells of the innate immune response, cells of adaptive immunity like the different classes of T and B lymphocytes have been shown to express a variety of TLRs33; however, at substantially lower magnitude than innate immune cells36. Furthermore, when interpreting these data, it also needs to be kept in mind that in a recently published study, it was shown that composition of PBMCs obtained from NAFLD patients differs from that of controls37 with lower frequencies being found for CD3+ and CD8+ T cells, CD56dimNK cells and MAIT cells but elevated frequencies of CD4+ T cells and Th2 cells. To what extend these differences added to the differences or lack of induction of TLRs in NALFD patients found in the present study needs to be clarified in future studies.
Expression of TLR1 in PBMCs of NAFLD patients and healthy controls was related to a higher relative abundance of the gram-positive bacterial genus Holdemanella and a lower relative abundance of Gemmiger and Ruminococcus in stool. Results of in vitro studies suggest that certain bacterial strains can alter expression of several TLRs in epithelial cell lines38; however, effects on the expression of TLR1 mRNA were limited in these studies. Studies assessing the effects of changes of bacterial composition on TLR expression in PMBCs with respect to certain bacteria to our knowledge are so far lacking. Also, if and how Holdemanella, Gemmiger or Ruminococcus might have added to the induction of TLR1 mRNA expression in PBMCs in the present study have to be determined in future studies.
Taken together, results of the present study suggest that intestinal microbiota, and herein, apperently specific genus like Holdemanella and bacterial toxins are positively related to TLR1 expression in PBMCs of NAFLD patients and that expression of TLR1 can be induced by both LPS and LTA. However, results by no means preclude that other TLRs in PBMCs may also be altered in patients with NAFLD as in the present study, only patients with fatty liver or NASH were studied. Rather, our results suggest that in patients with NAFLD, certain TLRs, and herein, probably especially TLR1 are induced in PBMCs and that this might also reflect elevated levels of certain bacterial toxins in peripheral blood.
It has been shown before by us and others that a genetic deletion or specific inhibition of certain TLRs, e.g. TLR4 and TLR9, may dampen or even abolish the development of NAFLD39–41 while for others like TLR2 was even found that a deletion may even exacerbate the development of NAFLD in mice42. In the present study, we found that the genetic deletion of TLR1 in mice fed a FFC is associated with a marked protection against the development of NAFLD. Indeed, results suggest that in livers of TLR1−/− mice with beginning inflammatory alterations were dampened. It has been suggested before, that TLR1-dependent signaling cascades are involved in mediating inflammatory response to various bacteria derived stimuli. Whether the protective effects of the loss of TLR1 in FFC-fed mice found in the present study are related to a lower sensitivity to LTA or LPS or both remains to be determined in future studies. Also, as we used whole body knockout mice and the yield of PBMCs of FFC-fed mice was too low to perform a statistical analysis, the results obtained in mice can only in part be related to the findings in humans and the question if an induction of TLR1 in PBMCs in NAFLD patients impacts the development of the disease will have to be addressed in future studies. Still, together with the results of others, results of the present study add further support to the role of bacterial toxins and TLR signaling in the development of NAFLD10,43.
The present study has some limitations. Specifically, the number of stool samples for analysis of the microbial communities in the control group is rather low (controls: n =
= 9). This has to be taken into consideration when interpreting the results of the present study. Despite this shortcoming, our findings that diversity of intestinal microbiota of healthy normal weight subjects differs from that of NAFLD patients is in line with those of others44. Although, while not reaching the level of significance (Fisher’s exact test p
9). This has to be taken into consideration when interpreting the results of the present study. Despite this shortcoming, our findings that diversity of intestinal microbiota of healthy normal weight subjects differs from that of NAFLD patients is in line with those of others44. Although, while not reaching the level of significance (Fisher’s exact test p =
= 0.0635), gender differences might have impacted the results of the present study. Indeed, gender has been shown to affect the development and progression of NAFLD, with women have a lower risk to develop NAFLD but a higher risk in progression of the disease than men45. In addition, as the yield of isolated PBMCs and resulting RNA of FFC-fed mice and their respective controls was too low, it was not possible to perform a proper statistical analysis of the data. Accordingly, the question if Tlr1 mRNA expression in PBMCs is also altered in FFC-fed mice suffering from NAFLD remains to be determined.
0.0635), gender differences might have impacted the results of the present study. Indeed, gender has been shown to affect the development and progression of NAFLD, with women have a lower risk to develop NAFLD but a higher risk in progression of the disease than men45. In addition, as the yield of isolated PBMCs and resulting RNA of FFC-fed mice and their respective controls was too low, it was not possible to perform a proper statistical analysis of the data. Accordingly, the question if Tlr1 mRNA expression in PBMCs is also altered in FFC-fed mice suffering from NAFLD remains to be determined.
Taken together, results of the present study further bolster the hypothesis that changes in intestinal microbiota composition in patients with NAFLD are associated with alterations of intestinal barrier function as well as increased bacterial endotoxin levels. Our results also suggest that contrary to the findings in liver tissue, in peripheral blood cells obtained from patients with NAFLD, only expression of TLR1 mRNA is increased and that this may depend upon LTA blood levels. Further studies are needed to determine clinical implications of these alterations, especially, in regard to disease progression. Furthermore, results of the present study also suggest that TLR1 may also be a critical factor in the development of NAFLD and that a genetic deletion of this receptor dampens the development of NAFLD; however, it remains to be determined, if these beneficial effects depend upon a decreased sensitivity towards LTA or other bacterial toxins and if effects alike are also found in humans.
Methods
Study subjects and design
A total of 37 patients with known NAFLD as diagnosed by ultrasound according to current guidelines and with no relevant alcohol intake (defined as <
< 20 g/d for women and
20 g/d for women and <
< 30 g/d for men) as well as 15 age-matched normal weight disease-free controls were recruited at the outpatient hepatology clinic of the University Medical Centre of the Johannes Gutenberg-University, Mainz, Germany and the Department of Nutritional Sciences, Vienna, Austria. The `Ethikkommission der Landesaerztekammer Rheinland-Pfalz´ (Germany) and the ethics committee of the Medical University Vienna (Austria) approved the studies in accordance with the Helsinki II Declaration and all patients and controls enrolled gave their written informed consent to participate in the study. NAFLD patients were part of the prospectively enrolling NUCES-NASH study (NCT02366052; Nutritional Counseling vs. Nutritional Supplements for NASH—a Randomized Prospective, Open Label Pilot Study) and the ENDO-META study (NCT03482284). None of the patients or controls followed any dietary restrictions or took antibiotics four weeks prior or at sample collection. Patients with NAFLD were included in the NUCES-NASH study when they had elevated M30 antigen levels (cut-off:
30 g/d for men) as well as 15 age-matched normal weight disease-free controls were recruited at the outpatient hepatology clinic of the University Medical Centre of the Johannes Gutenberg-University, Mainz, Germany and the Department of Nutritional Sciences, Vienna, Austria. The `Ethikkommission der Landesaerztekammer Rheinland-Pfalz´ (Germany) and the ethics committee of the Medical University Vienna (Austria) approved the studies in accordance with the Helsinki II Declaration and all patients and controls enrolled gave their written informed consent to participate in the study. NAFLD patients were part of the prospectively enrolling NUCES-NASH study (NCT02366052; Nutritional Counseling vs. Nutritional Supplements for NASH—a Randomized Prospective, Open Label Pilot Study) and the ENDO-META study (NCT03482284). None of the patients or controls followed any dietary restrictions or took antibiotics four weeks prior or at sample collection. Patients with NAFLD were included in the NUCES-NASH study when they had elevated M30 antigen levels (cut-off: >
> 200 to 800 U/L) and were diagnosed with hepatic steatosis by ultrasound or histologically confirmed NASH. The degree of hepatic steatosis was classified into mild (grade 1), moderate (grade 2) or severe (grade 3) according to a semi-quantitative scoring system46 in a standardized manner by a trained hepatologist. The term NAFLD was used to describe the NAFLD patients throughout the manuscript as liver histology was not accessible in all patients. Data presented were obtained at the screening visit of fasted patients and controls.
200 to 800 U/L) and were diagnosed with hepatic steatosis by ultrasound or histologically confirmed NASH. The degree of hepatic steatosis was classified into mild (grade 1), moderate (grade 2) or severe (grade 3) according to a semi-quantitative scoring system46 in a standardized manner by a trained hepatologist. The term NAFLD was used to describe the NAFLD patients throughout the manuscript as liver histology was not accessible in all patients. Data presented were obtained at the screening visit of fasted patients and controls.
Animal experiments
For all animal experiments, female 8–10 weeks old TLR1−/− and C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME, USA and Janvier SAS, Le Genest-Saint-Isle, France) were housed in a specific-pathogen-free barrier facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. All experiments were carried out under controlled conditions with mice having free access to tap water at all times. All procedures were approved by the local institutional animal care and use committee (`Regierungspraesidium Stuttgart´ and `Federal Ministry Republic of Austria Education, Science and Research´) and animals were handled in accordance to the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes and carried out in compliance with the ARRIVE guidelines. Mice were adapted to a liquid control diet (C; 69E% carbohydrates, 12E% fat, 19E% protein; Ssniff, Soest, Germany) and then randomly assigned to the different feeding groups. For the isolation of PBMCs, wild-type mice (n =
= 8) were pair-fed the C diet or a fat-, fructose- and cholesterol-rich diet (FFC; 60E% carbohydrates, 25E% fat, 15E% protein with 50% wt/wt fructose and 0.16% wt/wt cholesterol; Ssniff, Soest, Germany) for six weeks after the adaption to the liquid diet. To determine the role of TLR1 in the development of NAFLD, TLR1−/− (n
8) were pair-fed the C diet or a fat-, fructose- and cholesterol-rich diet (FFC; 60E% carbohydrates, 25E% fat, 15E% protein with 50% wt/wt fructose and 0.16% wt/wt cholesterol; Ssniff, Soest, Germany) for six weeks after the adaption to the liquid diet. To determine the role of TLR1 in the development of NAFLD, TLR1−/− (n =
= 5–6/group) and wild-type mice (n
5–6/group) and wild-type mice (n =
= 6/group) were either pair-fed the FFC or the C diet for eight weeks. As problems were encountered with breeding knockout mice and one mouse was lost when collecting fasting blood samples, it was only possible to analyze samples of n
6/group) were either pair-fed the FFC or the C diet for eight weeks. As problems were encountered with breeding knockout mice and one mouse was lost when collecting fasting blood samples, it was only possible to analyze samples of n =
= 5–6 TLR1−/− mice. For pair-feeding, caloric intake was adjusted daily to the group with the lowest caloric intake. Body weight was assessed weekly. After six weeks of feeding, TLR1−/− and wild-type mice were fasted for 6 h and blood was taken from retrobulbar venous plexus under isoflurane anesthesia. Fasting blood glucose levels were assessed with a glucometer (Bayer Consumer Care AG, Basel, Switzerland).
5–6 TLR1−/− mice. For pair-feeding, caloric intake was adjusted daily to the group with the lowest caloric intake. Body weight was assessed weekly. After six weeks of feeding, TLR1−/− and wild-type mice were fasted for 6 h and blood was taken from retrobulbar venous plexus under isoflurane anesthesia. Fasting blood glucose levels were assessed with a glucometer (Bayer Consumer Care AG, Basel, Switzerland).
Clinical measures, non-invasive scores of advanced hepatic fibrosis and vibration controlled transient elastography (VCTE)
Fasting blood samples were obtained from all NAFLD patients and controls to assess ALT, AST and γ-GT activity as well as triglycerides, total cholesterol, LDL and HDL cholesterol, CRP, glucose, insulin and uric acid concentration (Routine lab of the University Medical Centre of the Johannes Gutenberg-University, Mainz, Germany and IhrLabor, Vienna, Austria). In addition, concentrations of leptin (Hölzel Diagnostika Handels GmbH, Cologne, Germany), adiponectin (TECOmedical AG, Sissach, Switzerland) and PAI-1 (LOXO GmbH, Dossenheim, Germany) were measured with commercially available ELISA kits according to the manufacturers’ instructions. Type 2 diabetes, hypertension, hyperlipidemia and the metabolic syndrome were defined according to the definitions of the Joint Scientific Statement for Harmonizing the Metabolic Syndrome47. In NAFLD patients, NAFLD fibrosis score with age-adjusted cut-offs, APRI and Fib-4 score were calculated to predict advanced fibrosis as published48. Furthermore, VCTE was performed using Fibroscan 402 (Echosens, Paris, France) as part of the standard liver work-up by trained study coordinators using a standardized protocol in NAFLD patients. According to published findings, a cut-off of > 12.0 kPa was used to predict advanced fibrosis49,50.
12.0 kPa was used to predict advanced fibrosis49,50.
Isolation of PBMCs
PBMCs were isolated from whole blood specimen of humans and mice as described in detail previously51. In brief, whole blood was mixed with PBS and gently layered onto histopaque-1077 (Sigma-Aldrich Handels GmbH, Vienna, Austria). After density gradient centrifugation, PBMCs were collected from the plasma/histopaque interface. PBMCs were either frozen or lysed with Trizol (peqGOLD, Trifast, Peqlab, Germany) until further analyses or subsequent RNA isolation.
Stimulation of PBMCs ex vivo
Human PBMCs were stimulated with 100 ng/ml LPS (serotype O55:B5; Sigma-Aldrich, Steinheim, Germany) or 10 µg/ml LTA (Sigma-Aldrich, Steinheim, Germany) for 24 and 48 h, respectively. Cells were lysed in Trizol (peqGOLD TriFast, peqlab, Erlangen, Germany) and RNA was isolated.
Markers of intestinal permeability
Bacterial endotoxin and LTA levels in mouse and human plasma were assessed using commercially available assays (Charles River, Germany and Novatein Biosciences, MA, USA). Zonulin and citrulline blood concentrations were assessed using commercially ELISAs (Immundiagnostik AG, Bensheim, Germany and Hölzel Diagnostika Handels GmbH, Cologne, Germany) following the instructions of the manufacturer.
Scoring of liver histology and clinical markers in mice
Mouse liver sections (4 µm) of paraffin-embedded tissue were stained with hematoxylin and eosin to evaluate liver histology using NAS according to Kleiner et al.52. Activities of ALT and AST were determined in plasma in the routine laboratory of the University Hospital of Jena, Germany (Architect, Abbott, Wiesbaden, Germany).
RNA extraction and real-time RT PCR
RNA was isolated from frozen mouse and human PBMCs and mouse liver tissue using Trizol (peqGOLD TriFast, Peqlab, Germany) and reverse transcribed using a commercially available cDNA synthesis kit (Promega, Madison, WI, USA). Primers designed using the software Primer 3 are shown in Table S3. Real-time PCR was carried out and calculations were performed as previously detailed51.
TBARS assay
To determine TBARS, formed as a byproduct of lipid peroxidation53, liver tissue was homogenized in RIPA buffer supplemented with protease inhibitors. After precipitation with 10% trichloroacetic acid, supernatant was incubated with thiobarbituric acid for 10 min at 95 °C. Concentration of TBARS was measured at 532 nm with a photometer (SpectraMax, Molecular Devices, San Jose, CA, USA).
Illumina amplicon sequencing of fecal microbiota
Fecal samples from NAFLD patients (n =
= 21) and controls (n
21) and controls (n =
= 9) were analysed. All other patients and controls were either incompliant with sample collection or failed to transport samples appropriately to the laboratory. Bacterial DNA was extracted using FastDNA SPIN Kit for Soil (MP Biomedicals, Solon, OH, USA) following the manufacturer's instructions and Illumina amplicon sequencing of fecal microbiota was carried out as detailed previously54–56. In brief, the V1-2 region of 16S rRNA gene was amplified with a two-step PCR using Taq PrimeSTAR HS DNA (Clontech Laboratories, Mountain View, CA, USA). No negative control corrections were applied as PCR products were discarded if there was any evidence of product formation in the negative control when being checked on an agarose gel. Amplicons were purified and normalized using the SequalPrep Normalization Plate Kit (Thermo Fisher Scientific, Waltham, MA, USA). Samples were sequenced on an Illumina MiSeq platform using 250 bp paired-end sequencing chemistry. An average of 39.432
9) were analysed. All other patients and controls were either incompliant with sample collection or failed to transport samples appropriately to the laboratory. Bacterial DNA was extracted using FastDNA SPIN Kit for Soil (MP Biomedicals, Solon, OH, USA) following the manufacturer's instructions and Illumina amplicon sequencing of fecal microbiota was carried out as detailed previously54–56. In brief, the V1-2 region of 16S rRNA gene was amplified with a two-step PCR using Taq PrimeSTAR HS DNA (Clontech Laboratories, Mountain View, CA, USA). No negative control corrections were applied as PCR products were discarded if there was any evidence of product formation in the negative control when being checked on an agarose gel. Amplicons were purified and normalized using the SequalPrep Normalization Plate Kit (Thermo Fisher Scientific, Waltham, MA, USA). Samples were sequenced on an Illumina MiSeq platform using 250 bp paired-end sequencing chemistry. An average of 39.432 ±
± 5.971 raw reads per sample were quality filtered, assembled and aligned using Mothur pipeline57. Reads were clustered at 97% identity into 697 OTUs. Only OTUs present at an average abundance higher than 0.5% and a sequence length >
5.971 raw reads per sample were quality filtered, assembled and aligned using Mothur pipeline57. Reads were clustered at 97% identity into 697 OTUs. Only OTUs present at an average abundance higher than 0.5% and a sequence length > 250 bp were considered for further analysis. The closest representative was manually identified using seqmatch from RDP58. Sequences were submitted to European Nucleotide Archive under the accession number PRJEB41058.
250 bp were considered for further analysis. The closest representative was manually identified using seqmatch from RDP58. Sequences were submitted to European Nucleotide Archive under the accession number PRJEB41058.
Statistical analysis
All data are presented as means ±
± standard error of mean (SEM). Bartlett’s test was applied to test homogeneity of variances and data were log-transformed if unequal before performing further statistical tests. For analysis age and gender distribution between NAFLD patients and controls, a Fisher’s exact test was performed. For comparison of data of NAFLD patients and controls as well as of C- and FFC-fed mice an unpaired t-test was used while a two-factorial analysis of variance (ANOVA) was used to identify statistical differences between wild-type and TLR1−/− mice fed C or FFC followed by Tukey’s post hoc test. P
standard error of mean (SEM). Bartlett’s test was applied to test homogeneity of variances and data were log-transformed if unequal before performing further statistical tests. For analysis age and gender distribution between NAFLD patients and controls, a Fisher’s exact test was performed. For comparison of data of NAFLD patients and controls as well as of C- and FFC-fed mice an unpaired t-test was used while a two-factorial analysis of variance (ANOVA) was used to identify statistical differences between wild-type and TLR1−/− mice fed C or FFC followed by Tukey’s post hoc test. P ≤
≤ 0.05 was defined to be significant.
0.05 was defined to be significant.
To analyze Illumina amplicon sequencing data PRIMER-E 7 (Plymouth Marine Laboratory, Plymouth, UK) was used as detailed before59. For analysis of similarity (ANOSIM), a one-way permutation-based hypothesis testing was used with differences defined as significant if p ≤
≤ 0.05. Similarity percentage analysis (SIMPER) was used as detailed by Clarke and Warwick59 to calculate the percentage of similarity and dissimilarity and to determine which OTUs contribute to differences found in NAFLD patients and controls. Samples were standardized by total and a similarity matrix was created using Bray–Curtis coefficient60. Processed reads were imported to Calypso 8.84 for data analysis and visualization. The recommended program settings were used for standard filters for low sequence reads and Hellinger transformation (square root of total sum normalization). Hierarchical clustering with Euclidean distance and ordination of the community structures were visualized using PCoA and principal component analysis (PCA), CCA and RDA. To analyze the diversity indices, samples were rarefied to a read depth of 16.386 and an ANOVA was used to identify statistical differences between NAFLD patients and healthy controls. LEfSe was used to determine the bacteria most likely to explain differences between NAFLD patients and controls. Differences in the abundance of OTUs of interest between groups were evaluated using the unpaired Welch t-test.
0.05. Similarity percentage analysis (SIMPER) was used as detailed by Clarke and Warwick59 to calculate the percentage of similarity and dissimilarity and to determine which OTUs contribute to differences found in NAFLD patients and controls. Samples were standardized by total and a similarity matrix was created using Bray–Curtis coefficient60. Processed reads were imported to Calypso 8.84 for data analysis and visualization. The recommended program settings were used for standard filters for low sequence reads and Hellinger transformation (square root of total sum normalization). Hierarchical clustering with Euclidean distance and ordination of the community structures were visualized using PCoA and principal component analysis (PCA), CCA and RDA. To analyze the diversity indices, samples were rarefied to a read depth of 16.386 and an ANOVA was used to identify statistical differences between NAFLD patients and healthy controls. LEfSe was used to determine the bacteria most likely to explain differences between NAFLD patients and controls. Differences in the abundance of OTUs of interest between groups were evaluated using the unpaired Welch t-test.
Abbreviations
| γ-GT | Gamma glutamyl transferase |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| BMI | Body mass index |
| C | Control diet or healthy controls |
| CCA | Canonical correspondence analysis |
| CRP | C-reactive protein |
| FFC | Fat-, fructose- and cholesterol-rich diet |
| IRF3 | Interferon regulatory factor 3 |
| LDA | Linear Discriminant Analysis score |
| LEfSe | Linear discriminant analysis coupled with Effect Size |
| LPS | Lipopolysaccharide |
| LTA | Lipoteichoic acid |
| MYD88 | Myeloid differentiation primary response 88 |
| NAFLD | Non-alcoholic fatty liver disease |
| NAS | NAFLD activity score |
| NASH | Non-alcoholic steatohepatitis |
| OTUs | Operational taxonomic units |
| PAMP | Pathogen-associated molecular pattern |
| PAI-1 | Plasminogen activator inhibitor 1 |
| PBMCs | Peripheral blood mononuclear cells |
| PCoA | Principal Coordinates Analysis |
| RDA | Redundance analysis |
| TBARS | Thiobarbituric acid reactive substances |
| TIR | Toll/interleukin-1 receptor |
| TIRAP | TIR domain containing adaptor protein |
| TLRs | Toll-like receptors |
| Tnfα | Tumor necrosis factor alpha |
| TRAM | TRIF-related adaptor molecule |
| TRIF | TIR-domain-containing adapter-inducing interferon β |
| VCTE | Vibration controlled transient elastography |
Author contributions
I.B. and J.M.S. conceptualized and designed the experiments; A.Ba., A.N., A.H.A., A.Br., M.J.L.P, C.J.J., E.P. and A.C.S. performed the experiments; A.Ba., A.N., A.H.A. and A.C.S. analyzed the data; A.Ba., A.N. and I.B. wrote the paper. All authors reviewed the manuscript.
Funding
This work was funded in part by BMBF KN (FKZ 01GI1122H to I.B.). Open access funding provided by University of Vienna.
Data availability
Illumina amplicon sequences were submitted to European Nucleotide Archive under the accession number PRJEB41058.
Competing interests
J.M.S. serves as a consultant or advisory board member for Boehringer Ingelheim, Bristol-Myer Squibb, Echosens, Gilead, Genfit, Intercept, IQVIA, Madrigal, Nordic Bioscience, Novartis, Novo Nordisk, Pfizer, Siemens Healthineers. In addition, his institution has received grant support from Gilead Sciences and Yakult Deutschland. I.B. received funding of Yakult. All other authors declare no conflict of interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Anja Baumann and Anika Nier.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-97346-9.
References
Articles from Scientific Reports are provided here courtesy of Nature Publishing Group
Full text links
Read article at publisher's site: https://doi.org/10.1038/s41598-021-97346-9
Read article for free, from open access legal sources, via Unpaywall:
https://www.nature.com/articles/s41598-021-97346-9.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/113154430
Article citations
Targeting hepatic macrophages for non-alcoholic fatty liver disease therapy.
Front Cell Dev Biol, 12:1444198, 05 Sep 2024
Cited by: 0 articles | PMID: 39300994 | PMCID: PMC11410645
Review Free full text in Europe PMC
Gut-Liver-Pancreas Axis Crosstalk in Health and Disease: From the Role of Microbial Metabolites to Innovative Microbiota Manipulating Strategies.
Biomedicines, 12(7):1398, 24 Jun 2024
Cited by: 2 articles | PMID: 39061972 | PMCID: PMC11273695
Review Free full text in Europe PMC
Dynamic changes in immune cell populations by AXL kinase targeting diminish liver inflammation and fibrosis in experimental MASH.
Front Immunol, 15:1400553, 16 May 2024
Cited by: 0 articles | PMID: 38817615 | PMCID: PMC11137289
Toll-like receptors in health and disease.
MedComm (2020), 5(5):e549, 29 Apr 2024
Cited by: 4 articles | PMID: 38685971 | PMCID: PMC11057423
Review Free full text in Europe PMC
MASLD is related to impaired alcohol dehydrogenase (ADH) activity and elevated blood ethanol levels: Role of TNFα and JNK.
Redox Biol, 71:103121, 12 Mar 2024
Cited by: 0 articles | PMID: 38493749 | PMCID: PMC10957403
Go to all (12) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
BioProject
- (1 citation) BioProject - PRJEB41058
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Metformin attenuates the onset of non-alcoholic fatty liver disease and affects intestinal microbiota and barrier in small intestine.
Sci Rep, 9(1):6668, 30 Apr 2019
Cited by: 58 articles | PMID: 31040374 | PMCID: PMC6491483
Protective effect of quercetin on high-fat diet-induced non-alcoholic fatty liver disease in mice is mediated by modulating intestinal microbiota imbalance and related gut-liver axis activation.
Free Radic Biol Med, 102:188-202, 25 Nov 2016
Cited by: 178 articles | PMID: 27890642
Luteolin alleviates non-alcoholic fatty liver disease in rats via restoration of intestinal mucosal barrier damage and microbiota imbalance involving in gut-liver axis.
Arch Biochem Biophys, 711:109019, 31 Aug 2021
Cited by: 28 articles | PMID: 34478730
Role of gut microbiota and Toll-like receptors in nonalcoholic fatty liver disease.
World J Gastroenterol, 20(23):7381-7391, 01 Jun 2014
Cited by: 174 articles | PMID: 24966608 | PMCID: PMC4064083
Review Free full text in Europe PMC
Funding
Funders who supported this work.
Bundesministerium für Bildung und Forschung (1)
Grant ID: FKZ 01GI1122H

 1
1


