Abstract
Free full text

Relapsing-remitting CNS autoimmunity mediated by GFAP-specific CD8 T cells
Abstract
Multiple Sclerosis (MS) is an inflammatory disease of the CNS that causes the demyelination of nerve cells and destroys oligodendrocytes, neurons and axons. Historically, MS has been thought to be a CD4 T cell-mediated autoimmune disease of CNS white matter. However, recent studies have identified CD8 T cell infiltrates and gray matter lesions in MS patients. These findings suggest that CD8 T cells, and CNS antigens other than myelin proteins may be involved during the MS disease process. Here we show that CD8 T cells reactive to glial fibrillary acidic protein (GFAP), a protein expressed in astrocytes, can avoid tolerance mechanisms, and depending upon the T cell triggering event, drive unique aspects of inflammatory CNS autoimmunity. In GFAP-specific CD8 T cell receptor transgenic (BG1) mice, tissue resident memory-like CD8 T cells spontaneously infiltrate the gray matter and white matter of the CNS, resulting in a relapsing-remitting CNS autoimmunity. The frequency, severity and remissions from spontaneous disease are controlled by the presence of polyclonal B cells. In contrast, a viral trigger induces GFAP-specific CD8 T effector cells to exclusively target the meninges and vascular/perivascular space of the gray and white matter of the brain, causing a rapid, acute CNS disease. These findings demonstrate that the type of CD8 T cell-triggering event can determine the presentation of distinct CNS autoimmune disease pathologies.
Introduction
Multiple Sclerosis (MS) is an inflammatory T cell-mediated autoimmune disease of the Central Nervous System (CNS) that causes the demyelination of nerve cells and destroys oligodendrocytes, neurons and axons (1, 2). MS is thought to be primarily a CD4 T cell-mediated disease. Disease susceptibility linkage to MHC class II genes, the study of myelin-reactive CD4 T cells from MS patients and models of experimental autoimmune encephalomyelitis (EAE) clearly indicate that myelin-reactive CD4 T cells have a central role in MS disease pathogenesis (3–8). However, CD4 T cells are unlikely to be the sole mediators of disease pathogenicity as treatments specifically targeting these cells have failed to limit the rate of disease relapses or new lesion formation, whereas therapies which deplete or inhibit CNS infiltration of all lymphocyte subsets have been more successful (9–11).
Over the past several years, strong evidence has been accumulating to suggest that CD8 T cells also contribute to MS disease. Studies have shown that CD8 T cells are found in both white matter and gray matter MS plaques. In addition, these CD8 T cells are often oligoclonal, and can outnumber CD4 T cells regardless of the stage of activity or disease (2, 12–16). The antigen specificity of these CNS infiltrating CD8 T cells, however, remains unclear. In addition, the function of these T cells has been proposed to be either pathogenic or protective.
In support of CD8 T cells having a pathogenic role in the MS disease process, myelin-specific CD8 T cells have been isolated from MS patients that are capable of killing neuronal cells in vitro (17–21). In addition, MS disease susceptibility shows some genetic linkage to particular MHC class I alleles (22, 23). In animal models of CNS disease, CD8 T cells specific for myelin basic protein (MBP), myelin oligodendrocyte protein (MOG) and proteolipid protein (PLP) have been shown to be pathogenic (24–28). The clinical symptoms induced by CNS-reactive CD8 T cells can be diverse. Mice carrying activated MBP-specific CD8 T cells succumb to a non-paralytic, acute demyelinating CNS autoimmunity that is clinically and histologically different than those of classic CD4-EAE. These atypical-EAE disease pathologies have similarities to MS patients with upper motor neuron disease (24). Experiments with MOG and PLP-specific CD8 T cells, in contrast, induced CNS disease symptoms similar to classical EAE (25–28). These data suggest that myelin-specific CD8 T cells may contribute to some of the disease heterogeneity observed in MS patients.
In contrast to a pathogenic role, many studies have suggested CD8 T cells are suppressive to CNS disease. In animal models, early studies found that polyclonal CD8 T cells can limit disease severity and relapses of CD4 T cell-mediated EAE (29, 30). The ability of CD8 T cells to regulate CNS autoimmune disease may occur from CD8 T cells targeting activated CD4 T cells through the recognition of peptide displayed on MHC class I and Ib molecules, as well as by secreting IL-10 and other anti-inflammatory soluble mediators (5, 31–33). Consistent with these findings, CD8 T cell clones that can lyse myelin-specific CD4 T cells have been detected in MS patients (34–36), and longitudinal magnetic resonance imaging (MRI) analysis has shown a negative correlation between the percentage of Tc2 cytokine-producing CD8 T cells in the periphery of MS patients and the development of lesions (37). Thus, CD8 T cells like their CD4 counterparts can be pathogenic or be immuno-regulatory.
To contribute to the CNS autoimmune disease process, auto-reactive CD8 T cells have to avoid negative selection within the thymus and be exported to the peripheral T cell repertoire. Several CNS proteins, and in particular myelin proteins that are often the target of encephalogenic CD4 T cells, are predominately expressed behind the blood-brain barrier. The lack of or minimal expression of these CNS proteins within the thymus is thought to allow encephalogenic T cells to develop. However, some myelin epitopes are expressed and presented in the thymus, and developing T cells that are reactive to these ligands can be subject to thymic deletion or be skewed towards low avidity or suppressive responses (6, 38–41). Following export to the mature T cell repertoire, activation events result in CD8 T cells differentiating into a number of different effector and suppressor lineages, depending upon the TCR-pMHC interaction and the local inflammatory environment (42). Thus, some of the diverse contributions of CD8 T cells to CNS autoimmune disease may be a product of CD8 T cells that have differentiated into different effector or suppressor lineages, or target different CNS antigens.
Experiments presented here demonstrate that the clinical and histological features of CD8 T cell-mediated CNS disease are dependent upon how the CD8 T cells are activated and correlate with the CD8 T cells lineage that are present within the CNS. We observe that in C57BL/6 mice, CD8 T cells reactive to multiple CNS proteins are present in the mature T cell repertoire. For CD8 T cells that target GFAP, a protein expressed in astrocytes, we show that high avidity CD8 T cells that target a stable GFAP peptide/MHC complex can avoid tolerance mechanisms and when activated, induce white matter and gray matter CNS autoimmune pathology. Furthermore, spontaneous relapsing-remitting and chronic disease mediated by GFAP-specific CD8 T cells is associated with CD8 T cells with tissue resident memory-like phenotypes infiltrating the CNS parenchyma and is regulated by polyclonal B cells. In contrast, rapid acute disease following a viral trigger stems from GFAP-specific CD8 T cells with an effector and effector-memory lineage that are primarily located within the meninges and vascular/perivascular space, with minimal parenchymal infiltration. Differences in the composition and location of the lesions, as well as clinical symptoms created during spontaneous disease versus a viral induction, indicates that the triggering event that activates auto-reactive CD8 T cells can contribute to heterogeneity in CNS autoimmunity and clinical disease course.
Materials and Methods
Mice
C57BL/6, C57BL/6.SJL, Rag1−/−, µMT, Nur77-GFP and Gfap−/− mice were purchased from The Jackson Laboratory (Bar Harbor, ME). BG1 Tg mouse lines were created by injected TCR encoding plasmids directly into C57BL/6 oocytes. All mice were maintained in a pathogen-free environment in accordance with institutional guidelines in the Animal Care Facility at the University of Massachusetts Medical School.
Peptides, tetramers and Construction of recombinant viruses
The ability of 8–10mer peptide sequences from CNS proteins to bind MHC was evaluated using the consensus binding prediction implemented in the Immune Epitope Database (IEDB, www.iedb.org). All peptides were purchased from A&A lab, (San Diego, CA). Recombinant replication deficient human Ad5 adenovirus expressing the full length GFAP, MAG, MBP, MOG or PLP cDNA was constructed using the AdEasy XL Adenoviral Vector System (Stratagene). Recombinant vaccinia viruses expressing the full length GFAP, MAG, MBP, MOG or PLP cDNA, and protein truncations fused to green fluorescent protein (GFP) inserted into the thymidine kinase gene were constructed (43). H2-Db and H2-Kb tetramers presenting GFAP264–272 were constructed using published methods (44).
Isolation and characterization of the CD8 T cell clone and cloning of the BG1 TCR
4 week old C57BL/6 mice were infected i.p. with 2 × 107 pfu recombinant human Ad5 adenovirus or Vaccinia expressing full length GFAP, MAG, MBP, MOG or PLP. Following three weeks infection, 2 × 107 spleen and lymph node cells were isolated and stimulated in vitro with irradiated spleen cells loaded with 1µg/ml of a pool of five different peptides. Following one week in culture, responding T cells were cloned by limiting dilution with irradiated spleen cells pulsed with 1µg/ml peptide pools and 50U IL-2. Cultures that demonstrated T cell expansions were expanded by weekly re-stimulation and tested for specificity as readout by the ability to produce IFNγ in response to target cells loaded with GFAP peptides.
TCR Vβ chains were identified by staining with a set of Vβ specific antibodies (BD biosciences), the TCRα chains was identified by PCR analysis using a panel of TCR Vα primers that collectively amplify all TCR Vα gene families. GFAP-specific T cells were found to express TCRs carrying Vα4:Vβ9 and Vα5:Vβ9 sequences, MOG-specific T cells were found to express TCRs carrying Vα4:Vβ4, Vα8:Vβ4 and Vα10:Vβ11 sequences and MAG-specific T cells were found to express different Vα17:Vβ11 clonotypes.
The rearranged cDNAs for BG1 TCR Vα4.5 was cloned XhoI to BspEI using the primers: Vα4.5 sense:
GGGCTCGAGAAGATGGACTCTTCTCCAG
TCR Cα antisense: CTGGTACACAGCAGGTTCCGGATTCTGGATGT
The rearranged cDNAs for Vβ9.1 gene was cloned EcoRI to Bgl2 using the primers:
Vβ9.1 sense: TCGACGAATTCGAGAGGAAGCATGGATCCTAGACTTCTTTGCTG
TCR Cβ antisense: CTTGGGTGGAGTCACATTTCTCAGATCTTC
All the TCR genes were sequenced, and error-free full length cDNAs were subcloned into the human CD2 promoter transgene cassette for T cell–specific expression (45). TCR Tg mouse lines were established by injecting C57BL/6 oocytes with the TCR Tg plasmids.
Characterization of CD8 T cell specificity
The specificity of the T cell clones were assessed by the ability to produce IFNγ following antigen recognition. The CD8 T cell clones were re-stimulated for 5 days, then 3 × 105 CD8 T cells were incubated for 4 hr at 37°C with 1 × 105 BM-DCs infected at an MOI of 3 with either Vac:GFAP, MAG, MOG or Vac:Neg viruses, or pulsed with GFAP, MAG or MOG peptides in the presence of GolgiStop and GolgiPlug (1µl/ml BD biosciences). BM-DC were differentiated in vitro by growing BM with GM-CSF for seven days following (43). For analysis of IFNγ production, T cells were then surfaced stained with anti-CD8 and Thy1.2, washed, fixed in 2% formaldehyde (Fischer Scientific) and stained for intracellular IFNγ using a Cytofix/Cytoperm kit (BD biosciences) using manufacturer’s protocol. To clarify MHC restriction, CD8 T cell clones were tested for the ability to recognize GFAP, MAG or MOG peptides presented by BM-DC expanded from NOD mice (H2-Kd and H2-Db), or a fibroblast cell line isolated from H2-Kb−/−H2-Db−/− mice that was retrovirally transduced with either H2-Kb or H2-Db. Flow cytometry (LSRII, BD biosciences) of CD8 T cell responses was analyzed using FlowJo version 8.3 (TreeStar).
RMA-S Stabilization Assay
To detect ability of binding of peptides on MHC class I molecules, 5 × 105 RMA-S cells, which are antigen- processing defective T cell lymphoma derived from C57BL/6, were mixed with peptides at various concentrations, and incubated at 25 °C overnight. Cells were cultured at 37 °C for 2 hrs, washed and stained for MHC class I molecule surface expression using anti-H2-Kb-PE (AF6-885) and anti-H2-Db-FITC (KH95) purchased from BD biosciences.
Clinical Scoring Scale for Classical and Non-Classical CNS Autoimmunity
Classical EAE was scored on a scale of 0–5 following traditional scoring methods (46). Non-classical EAE symptoms were scored on a scale of 0–12 using a method that test for cerebellar dysfunction. Scores of 0–3 are given for balancing on a ledge, hind limb clasping, gait and kyphosis (47).
Isolation of T cells from CNS and analysis of lymphocyte populations
To isolate lymphocytes from the CNS, mice were deeply anesthetized with 125 mg/kg Ketamine and 10 mg/kg Xylazine, and perfused through the caudal vena cava with 40mls heparinized PBS. Brain and spinal cord were isolated, manually disassociated and passed through a 40µm cell strainer. Lymphocyte populations were isolated from single cell suspensions using a 30% and 70% percoll density gradient. Lymphocyte populations were cell surface stained and analyzed by flow cytometry. In some experiments, cells were fixed in 2% formaldehyde and analyzed by intracellular staining. To identify the cytokine profile, lymphocytes were stimulated in vitro with 50ng/ml PMA and 750ng/ml ionomycin, in the presence of Golgi stop/Golgi plug (1:1000) for 4 hours at 37°C and analyzed for intracellular cytokine expression.
Vac-GFAP induction of CNS autoimmunity
CD8 T cells (CD45.2+) were positively isolated from BG1 mice using magnetic-activated cell sorting (MACS). Titrating numbers of BG1 CD8 T cells were intravenously transferred into recipient C57BL/6.SJL (CD45.1+) followed by infected using 1 × 107 pfu Vac:GFAP on day 0. Mice were monitored for six weeks (only two weeks is shown in figure). Disease scores are reported based on atypical EAE symptoms. Transferred BG1 CD8 T cells in spleen and CNS were analyzed by flow cytometry on day 4, 6 and 14.
Histologic Analysis of Brain and Spinal Cords
For formalin-fixed paraffin sections, brain and spinal cord were dissected, fixed in 10% Millonig’s modified phosphate-buffered formalin for 48hr, sectioned, and embedded in paraffin wax. To ensure reproducibility, a rodent brain matrix (Electron Microscopy Sciences) was used for all brain sectioning. Six serial sections of five brain cross-sections, and three longitudinal sections (C1-6, T3-13, L3-cauda equina) per mouse were stained with hematoxylin and eosin (H&E), Luxol Fast Blue (LFB) or mAb specific for GFAP (Vector Laboratories). Sections from other major organs from these mice were stained with H&E.
For immunohistochemistry analysis, mice were deeply anesthetized and perfused with 40mls of ice cold heparinized PBS with 4% paraformaldehyde. Brain and spinal cords were dissected, cryopreserved in 10% sucrose for 2hrs, 20% sucrose for 2hrs and 30% sucrose overnight at 4 °C. Sectioned tissues were then frozen in Optimal Cutting Temperature compound (Tissue-Tek). The brain sections were cut, fixed in acetone and blocked with anti-CD16/32 antibody (2.4G2) followed by with 5% goat serum plus blocking buffer (PBS containing 2% bovine serum albumin, 0.1% Triton X-100 and 0.02% sodium azide). After washed, the sections were stained with the following antibodies in blocking buffer for 1 hr at room temperature plus overnight at 4°C: anti-CD4-FITC (RM4.5), anti-B220-APC (RA3-6B2), anti-CD11c-Biotin (N418) and anti-CD19-Biotin (eBio1D3) purchased from eBioscience; anti-CD3e-FITC (145-2C11), anti-CD3e-APC (145-2C11), anti-CD4- APC (RM4.5) purchased from BD biosciences; anti-CD8-APC (2.43) purchased from Bio X cell; Rat anti- GFAP (2.2B10) purchased from Vector Laboratories; goat anit-Rat IgG-Texas Red, Streptavidin-FITC and goat purchased from Molecular Probes. For preservation, the labeled sections were mounted in Prolong Gold (Molecular Probes) with or without DAPI that stains nuclei. Images were acquired on a Leica TCS SP5 II confocal microscope using HCX PL APO 63×/1.40–0.60 and HCX PL APO 20×/0.70 objective lenses. Signals were acquired with LAS AF software (Leica), and Photoshop CS4 (Adobe) was used for image processing.
Results
Isolation of CD8 T cells reactive to GFAP, MAG and MOG
To identify CNS-reactive CD8 T cells that avoid negative selection within the thymus and populate the T cell repertoire, C57BL/6 mice were infected with recombinant Vaccinia virus (Vac) or adenovirus (Ad) expressing the full-length cDNA of GFAP, MAG, MBP, MOG or PLP. Following three weeks, splenocytes from infected mice were re-stimulated in vitro with GFAP, MAG, MBP, MOG or PLP peptides that carry H-2b MHC class I binding motifs and cloned by limiting dilution. Using this protocol, we isolated CD8 T cell clones specific for GFAP264–272 (AASRNAELL) bound to H2-Db, MAG444–451 (VICTSRNL) bound to H2-Kb and MOG81–88 (VTLRIQNV) bound to H2-Kb (Fig. 1A–I). However, we were unable to identify MBP- or PLP-specific CD8 T cells from C57BL/6 mice. GFAP-specific CD8 T cells strongly reacted with APC presenting soluble GFAP264–272, with a EC50 value of 40nM, whereas MAG- and MOG-specific CD8 T cells showed a weaker response, with EC50 values of 3µM and 2µM, respectively (Fig. 1A, D, G). Correlating with the strong CD8 T cell response, the GFAP264–272 peptide was observed to stably bind H2-Db, as read out using an RMA-S stabilization assay (Fig. 1J). In contrast, both MAG444–451 and MOG81–88 epitopes were found to poorly stabilize H2-Kb (Fig. 1K). These data indicate that C57BL/6 mice carry CD8 T cells reactive to multiple CNS antigens, including strong avidity CD8 T cells that target a stable GFAP peptide/MHC complex.
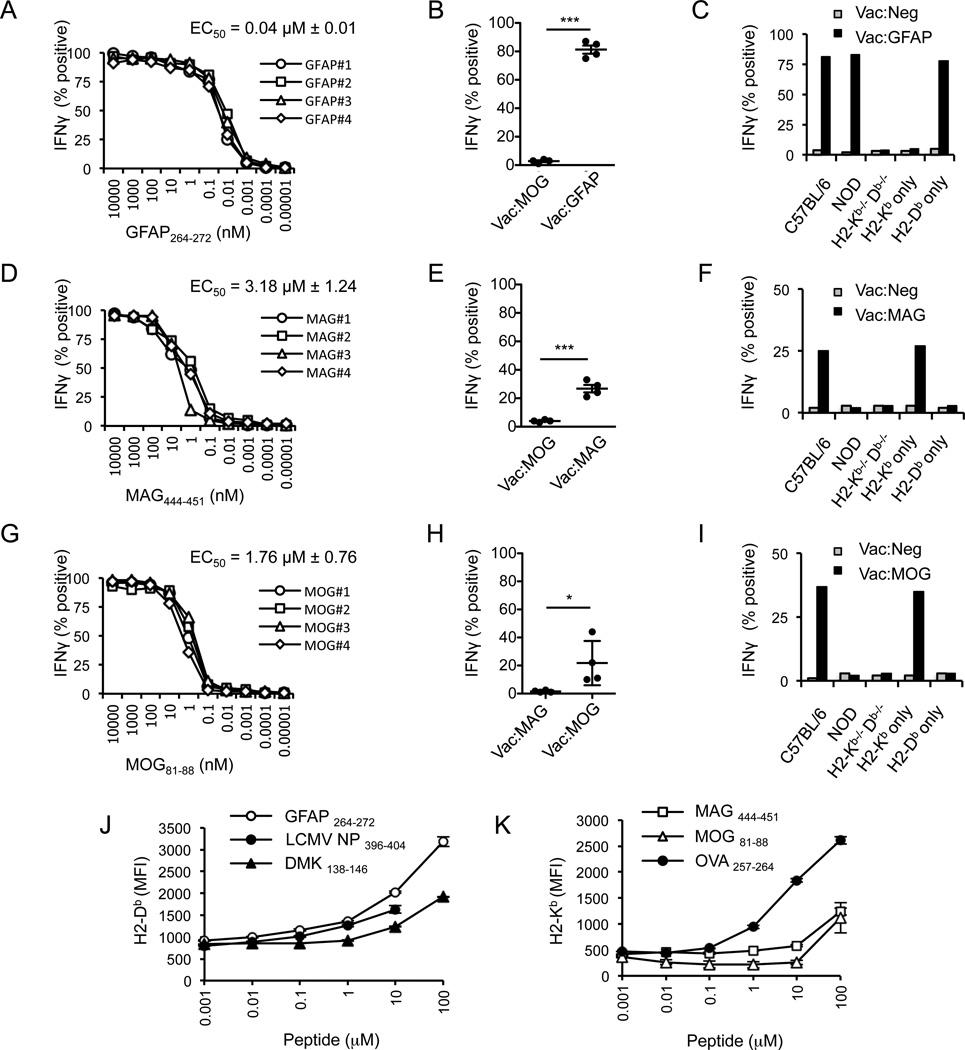
Isolation and characterization of CD8 T cell clone specific for GFAP, MAG and MOG. CD8+ T cells were isolated from spleen and lymph nodes of Ad-GFAP, MAG and MOG or Vac-GFAP, MAG and MOG infected C57BL/6 mice, re-stimulated with different peptides predicted to bind to either H2-Kb or H2-Db and cloned by limiting dilution. (A–C) Four different GFAP264–272-specific, (D–F) MAG444–451-specific or (G–I) MOG81-88-specific CD8 T cell clones were tested for the ability to produces IFNγ in response to APC presenting titrating concentrations of peptide antigen, the ability to recognize virally infected target cells, and to recognize target cells expressing only H2-Kb or H2-Db. (J) RMA-S cells were incubated overnight at 25 °C with different concentrations of (J) GFAP264–272, LCMV NP396–404 or DMK138–146, or (K) MAG444–451, MOG81–88, or OVA257–264 and RMA-S cells were then shifted to 37 degrees for 2 hours, and stained with antibodies specific for H2-Db or H2-Kb. Data shown are representative of at least 3 individual experiments.
Generation of BG1 TCR Tg mice specific for GFAP264–272
To identify how strong avidity CD8 T cells reactive to GFAP avoid tolerance mechanisms we constructed TCR Tg mice expressing the Vα4.5 and Vβ9.1 TCR chains from the GFAP-specific CD8 T cell clone, BG1. Several founder lines were created, each of which displayed a similar phenotype, and one line was chosen for comprehensive analysis.
GFAP is expressed within the brain and spinal cord and, at a lower level, in some peripheral tissues including the thymus, intestine and pancreas (48). Thus, it is possible that developing BG1 CD8 T cells could engage cells presenting the GFAP peptide and undergo deletion or inactivation. To determine if tolerance was occurring, CD8 T cells isolated from BG1 mice were compared to CD8 T cells isolated GFAP-deficient (Gfap−/−) BG1 mice, mice that do not express the self-antigen BG1 T cells recognize (Fig. 2). Central or peripheral tolerance was not observed in four week old BG1 mice; wild type (WT) and Gfap−/− BG1 mice have a similar thymic cellularity, produce similar numbers of CD8 single positive thymocytes and mature CD8 T cells that stain equivalently with H2-Db/GFAP264–272 tetramers (Fig 2A–D), and have a similar ability to produce TNFα and IFNγ in response to titrating amounts of GFAP264–272 peptide (Fig. 2E, F)).
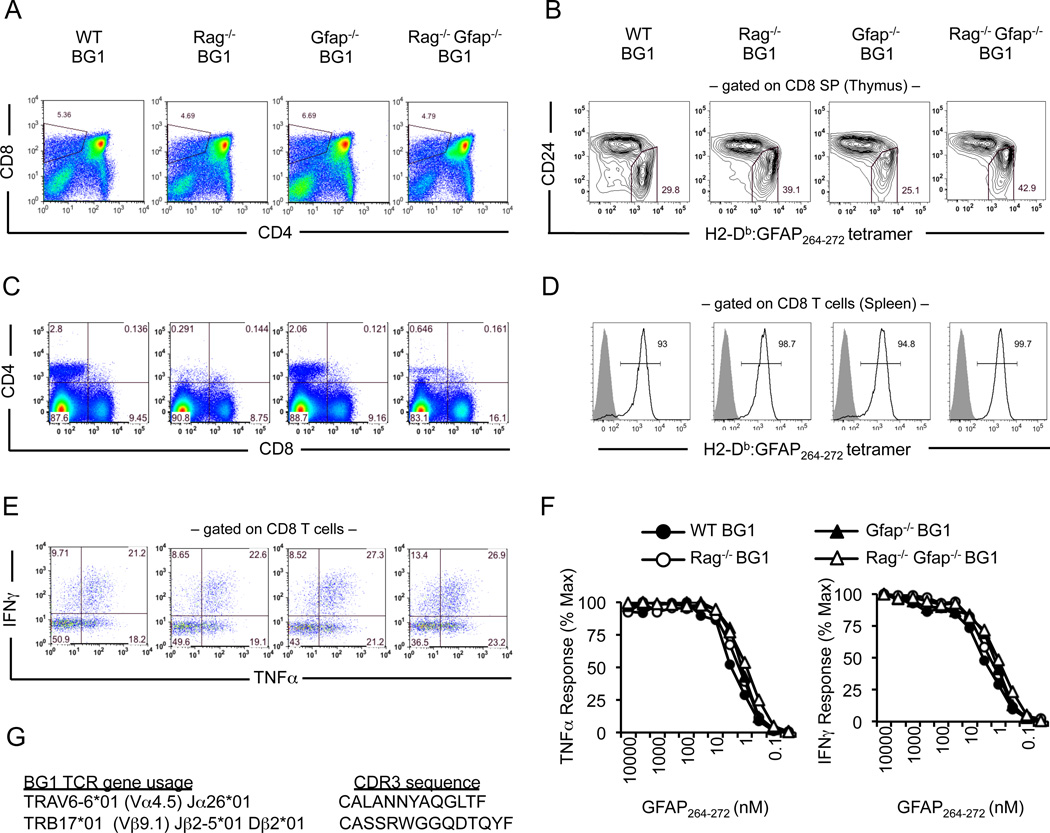
Development of GFAP-specific CD8+ T cells in BG1 mice. (A, B) Thymocytes or (C–F) splenocytes from 4 week old WT BG1, Rag−/− BG1, Gfap−/− BG1 and Rag−/−Gfap−/− BG1 mice were stained with indicated antibodies, including H2-Db:GFAP264–272 tetramer. Reactivity to H2-Db:GFAP262–272 tetramer is shown for (B) CD8 single positive thymocytes or (D) mature CD8+ T cells (black lined histogram). Filled gray histograms show H2-Db:GFAP262–272 tetramer staining of CD8 T cells isolated from C57BL/6 mice. (E, F) CD8 T cell splenocytes from BG1 mice produce IFNγ and TNFα following activation with APC presenting titrating concentration of GFAP264–272, shown in (E) for response at 1 µM GFAP264–272. Each result is representative of at least five mice per group, each experiment was performed at least twice. (G) Sequence of the BG1 TRAV6-6*01 (Vα4.5) TCRα chain and TRB17*01 (Vβ9.1) TCRβ chain.
Consistent with the hypothesis that BG1 CD8 T cells are primarily ignorant of GFAP within secondary lymphoid organs, BG1 CD8 T cells isolated from Gfap−/− mice did not undergo proliferation when transferred into GFAP-expressing C57BL/6 mice (Fig. 3A, B). BG1 CD8 T cells did strongly proliferate if recipient mice were infected with Vac:GFAP, indicating the transferred CD8 T cells did not become unresponsive to T cell activation (Fig. 3C, D). These data indicate that GFAP-reactive CD8 T cells that are not subject to overt thymic deletion or the development of unresponsiveness can develop in C57BL/6 mice. CD8 T cells were, however, found to spontaneously enter into the brain and spinal cord of BG1 mice. Within the CNS tissue, the majority of BG1 CD8 T cells are receiving signals through their TCR, as they strongly express the TCR signaling- dependent protein, Nur77 (49), as well as the activation markers CD69 and CD25 (Fig 3E, F). In contrast, BG1 CD8 T cells were not observed to generate strong TCR-mediated signals in peripheral lymphoid organs or non-CNS organs (Fig. 3E–H).
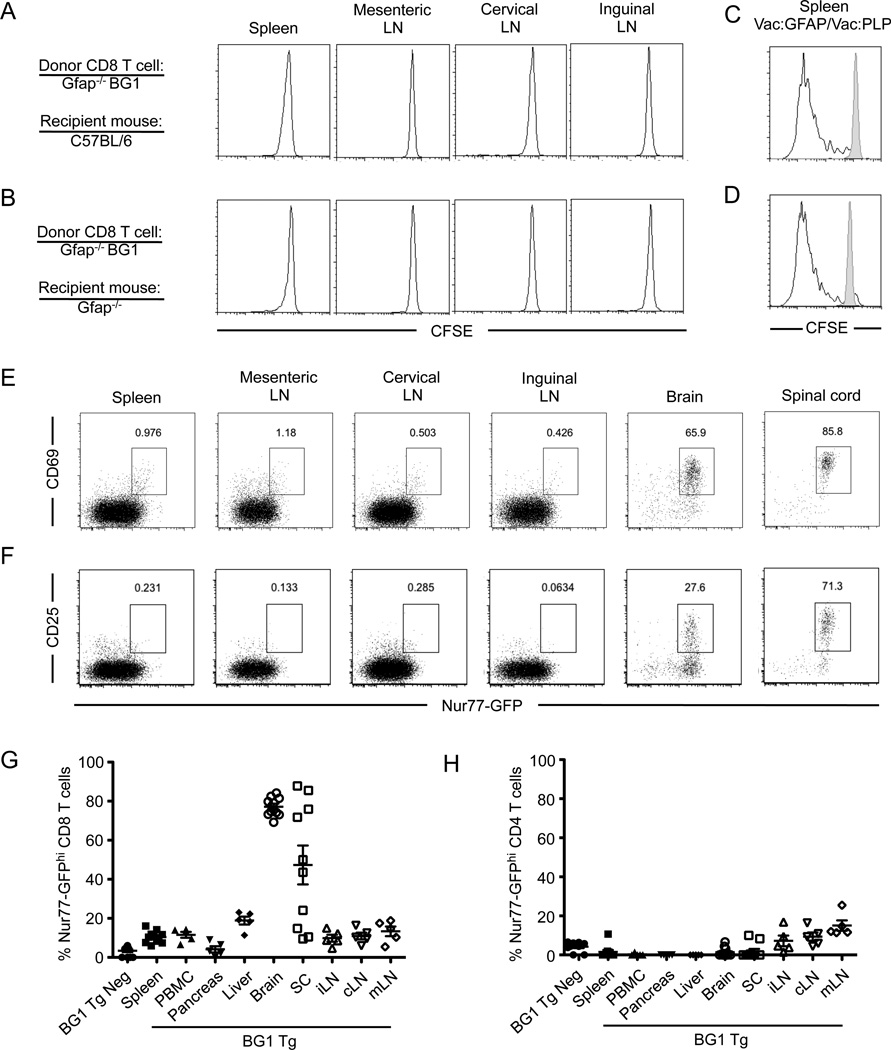
BG1 CD8 T cells are primarily ignorant of to endogenous GFAP antigen within the secondary lymphoid organs. (A) Naïve Gfap−/− CD45.2+ BG1 CD8 T cells were labeled with CFSE and adoptively transferred into Gfap+/+ CD45.1+ or (B) Gfap−/− CD45.1+ recipients. CD45.2+ CD8 T cells were analyzed for CFSE dilution three days post transfer, or (C, D) infected with Vac:GFAP (blacked lined histogram) or Vac:PLP (gray filled histogram) and analyzed three days after infection. (E) CD8 T cells from 8 week old BG1 TCR Tg mice carrying the TCR-signaling reporter transgene Nur77-GFP were analyzed for GFP expression and the activation markers CD69 or (F) CD25 within the secondary lymphoid organs and CNS. (G) Quantitation of Nur77-GFP expression in BG1 CD8 T cells and (H) CD4 T cells in lymphoid and non-lymphoid tissues. Results are representative of at least two independent experiments with three mice per group.
BG1 mice succumb to spontaneous relapsing-remitting CNS autoimmunity
To determine if BG1 mice maintain quiescence to GFAP over their lifetime, a cohort of BG1 mice on a C57BL/6 background (WT BG1), Rag1−/− BG1 and Gfap−/− BG1 mice were analyzed for clinical signs of CNS disease as they aged. We observed that BG1 mice do not maintain ignorance of GFAP as approximately 47% (44/93) of WT BG1 mice and 100% of (22/22) Rag−/− BG1 mice succumb to spontaneous clinical signs CNS autoimmunity by six months of age (Fig. 4A).
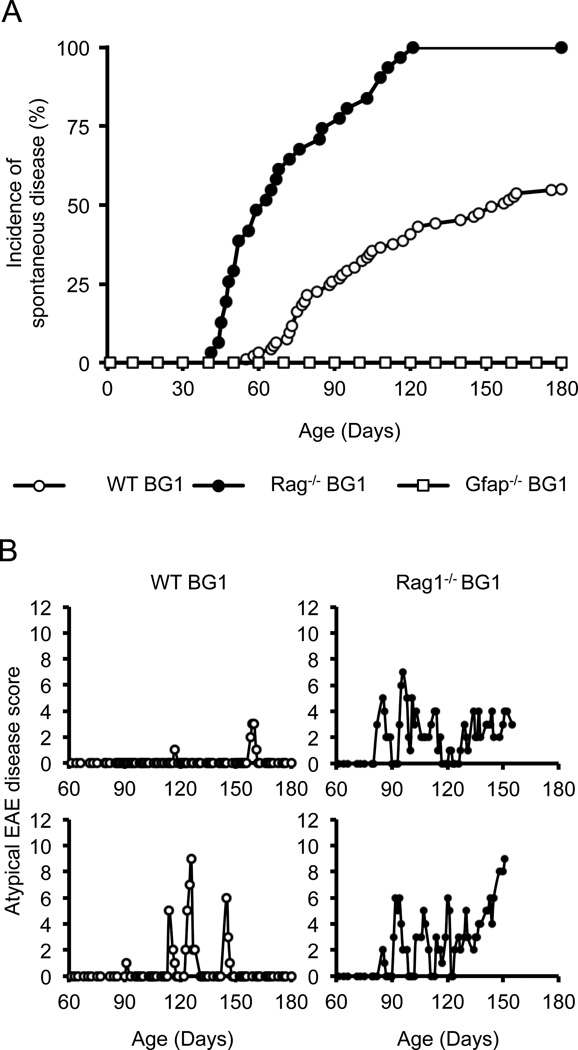
WT BG1 and Rag−/− BG1 mice succumb to spontaneous relapsing-remitting CNS autoimmunity. (A) Incidence of first clinical signs of spontaneous EAE-like symptoms. Disease incidences are statistically different between WT BG1 (n=93) and Rag−/− BG1 (n=22) mice (p=0.003, log-rank test). No Gfap−/− BG1 mice (n=15) developed signs of disease symptoms. (B) Two examples of a clinical disease course for WT BG1 (open circle) and (C) Rag−/− BG1 mice (filled circle). Note, some of the Rag−/− BG1 mice did not survive to 180 day-old because of progressive disease.
WT BG1 and Rag−/− BG1 mice developed two distinguishable forms of clinical symptoms (Table 1). The majority of diseased WT BG1 mice (34/44) and Rag1−/− BG1 mice (22/22) developed balancing defects, lethargy, uneven gait and ataxia (video S1). These symptoms are referred to as atypical disease, and were scored using a scale of 0–12 that tests for cerebellar dysfunction. Scores of 0–3 are given for balancing on a ledge, hind limb clasping, gait and kyphosis (47). Most diseased WT BG1 (27/44) and some Rag−/− BG1 mice (9/22) also succumbed to mild ascending flaccid paralysis, symptoms referred to as classical EAE. These mice were scored on a scale of 0–5, following classical EAE protocol. Some of the diseased WT BG1 mice (10/44) only displayed classical EAE symptoms (Table 1). In both WT BG1 and Rag1−/− BG1 mice, clinical symptoms began as episodic bouts of functional impairment, with many mice displaying severe CNS dysfunction (e.g., classical disease scores of 3, atypical scores of 6–8) and then remitting to unobservable functional defects (Fig. 3B, C). Rag1−/− BG1 mice developed more severe bouts of disease, and had more relapses than WT BG1 mice (Table 1). As Rag1−/− BG1 mice aged, (21/22) mice developed chronic disease (defined as at least 30 days of continual functional impairment), with a small subset further progressing to morbidity (4/22). In contrast, only (1/44) WT BG1 mice converted to chronic disease.
Table I
Spontaneous CNS disease among different genetic backgrounds of BG1 mice.
| WT BG1 | Rag−/−BG1 | MHCII+/− BG1 | MHCII−/− BG1 | µMT+/− BG1 | µMT−/− BG1 | |
|---|---|---|---|---|---|---|
| Spontaneous disease incidence (%) | 47 (44 / 93) | 100 (22 / 22) | 12 (2 / 17) | 30 (8 / 27) | 48 (10 /21) | 96 (24 / 25) |
| Male (%) | 47 (19 / 40) | 100 (9 / 9) | 13 (1 / 8) | 29 (2 / 7) | 44 (4 / 9) | 100 (15 / 15) |
| Female (%) | 47 (25 / 53) | 100 (13 / 13) | 13 (1 / 8) | 30 (6 / 20) | 50 (6 / 12) | 90 (9 / 10) |
| Days of onset | 113 | 77 | 99 | 72 | 98 | 104 |
| Classical EAE only (%) | 23 (10 / 44) | 0 (0 / 22) | 0 (0 / 2) | 0 (0 / 8) | 0 (0 / 10) | 0 (0 / 24) |
| Atypical EAE only (%) | 39 (17 / 44) | 59 (13 / 22) | 50 (1 / 2) | 63 (5 / 8) | 90 (9 / 10) | 71 (17 / 24) |
| Both classical and atypical EAE (%) | 39 (17 / 44) | 41 (9 / 22) | 50 (1 / 2) | 38 (3 / 8) | 10 (1 / 10) | 29 (7 / 24) |
| Relapse-Remitting EAE (%) | 98 (43 / 44) | 64 (14 / 22) | 0 (0 / 2) | 63 (5 / 8) | 10 (1 / 10) | 4 (1 / 24) |
| # of relapses 60 days post onset | 1.1 | 1.7 ** | 1.0 | |||
| Ave. Duration of relapse (days) | 3.1 | 5.0 *** | 5.3 | |||
| Ave. Max severity of classical EAE | 1.0 | 1.1 | 1.0 | |||
| Ave. Max severity of atypical EAE | 3.9 | 5.8 *** | 4.2 | |||
| Relapse-Remitting and Late-chronic EAE (%) | 0 (0 / 44) | 59 (13 / 22) | 0 (0 / 2) | 38 (3 / 8) | 0 (0 / 10) | 4 (1 / 24) |
| Chronic EAE only (%) | 2 (1 / 44) | 36 (8 / 22) | 50 (1 / 2) | 38 (3 / 8) | 50 (5 / 10) | 83 (20 / 24) |
Each strains of BG1 mice were monitored for clinical symptoms of classical or atypical EAE 5 days a week over 180 days after birth. The two distinct phenotypes were considered only when they were observed more than 2 days in a row to prevent the positive-false cases. The observed diverse clinical phenotypes were classified as 4 groups; acute, relapse-remitting, primary chronic (chronic only) and secondary chronic disease (relapse-remitting followed by chronic disease). Chronic EAE was defined as atypical disease lasting at least 20 days. Chronic phase of classical EAE disease was not observed in BG1 mice. Probability values (P) from Mann-Whitney test are presented when compared to the data of WT BG1 mice.
GFAP-specific CD8 T cells infiltrate the brain of BG1 mice
The neurological impairments observed in diseased BG1 mice indicated that pathogenic CD8 T cells were infiltrating the CNS. To identify the time frame in which CD8 T cells enter into the CNS, we analyzed cellular infiltrates in BG1 mice at different ages. In WT BG1 mice and Rag−/− BG1 mice, CD8 T cells were found within the brain at 30 days of age, and are present throughout the life (Fig. 5A). Because not all WT BG1 mice succumb to spontaneous clinical symptoms, T cell trafficking to the CNS is not the sole arbiter of whether overt clinical disease occurs. In addition to CD8 T cells, CD4 T cells and B cells were found within the brain of WT BG1 mice at all ages (Fig. 5B, C). Similar lymphocyte populations were observed within the spinal cord, albeit at a lower frequency than in the brain (Fig. 5D–F). These frequencies correspond to a 10-fold increase in T cells and 100-fold increase in B cells that infiltrate the brain as compared with the spinal cord, on a per weight basis. These lymphocyte accumulations within the CNS require CD8 T cell recognition of GFAP, as Gfap−/− BG1 mice carry few T cells or B cells within the brain.
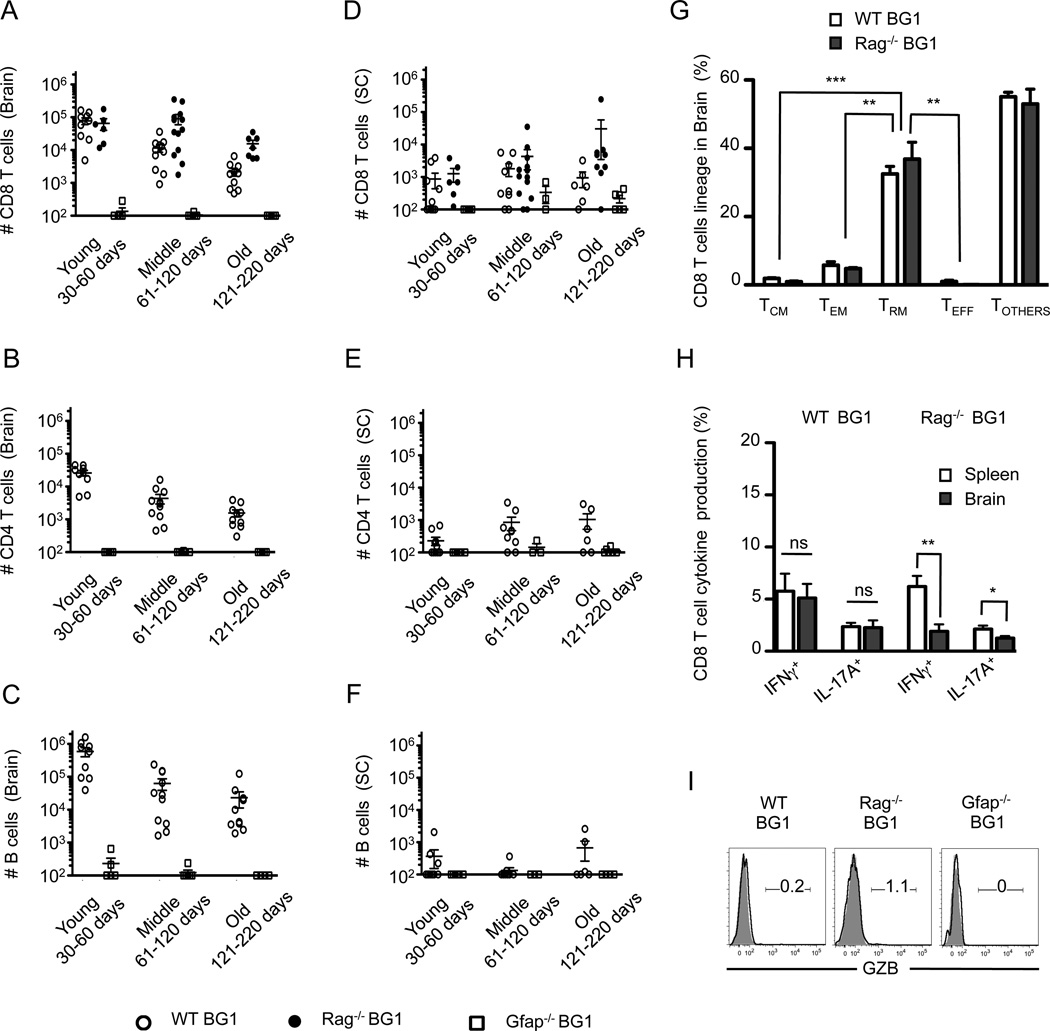
Brain-infiltrating BG1 CD8+ T cells enter into the CNS of young mice and have memory-like phenotypes with limited inflammatory and cytotoxic function. (A–C) Absolute numbers of lymphocytes in the brain and (D–F) spinal cord were determined for three different age groups. CD8 T cells (Thy1.2+ CD8+ CD4- B220-), CD4 T cells (Thy1.2+ CD8- CD4+ B220-) and B cells (Thy1.2- CD8- CD4- B220+) were identified by flow cytometry. (G) BG1 CD8 T cell subsets within the brain were determined by the expression of CD62L, CD44, CD127, KLRG1, CD103 and CD69 as shown in Fig. S2. CD8 T cells were defined as TCM (CD62L+ CD44+ KLRG1-), TEM (CD62L- CD44+ KLRG1-) and TEFF (CD62L- CD44+ KLRG1+) or TRM (CD44+ CD69+ CD103+). (H) BG1 CD8 T cells within the brain of 45–60 day old BG1 mice poorly secrete IFNγ and IL-17A, and (I) lack granzyme B production. Data shown are from at least six individual mice per group. Error bars in graphs represent SEM. *; P=<0.05, **; P=<0.01, ***; P=<0.001, ns; Not Significant.
To identify the CD8 T cell effector populations that target the CNS during spontaneous CNS disease, the lineage and effector function of brain-resident CD8 T cells was determined. The CD8 T cells present within the CNS of WT BG1 mice and Rag−/− BG1 mice show a similar, mixed expression of CD44 and CD62L, and a coincident lack of KLRG1 or CD127 expression (Fig. 5G, S2). In addition, a large frequency of the CD8 T cells residing within the brain express the activation marker, CD69, and the αE integrin, CD103. Based on these phenotypic markers, many of the brain-resident CD8 T cells resemble anti-viral tissue-resident memory (TRM) cells that populate peripheral tissues following viral challenges (Fig. 5G) (50, 51). Functionally, however, only low frequencies of CD8 T cells within the CNS are capable of producing IFNγ, IL-17 or granzyme B (GZB), indicating that many of the BG1 CD8 T cells present within the brain are not classic effector CD8 T cells (Fig. 5H, I). Collectively, these data indicate that BG1 CD8 T cells that spontaneously enter into the brain recognize cells presenting GFAP264–272, with many adapting to the brain environment without gaining inflammatory cytokine expression or cytotoxic effector functions.
BG1 CD8 T cells target the cerebellum, mid-brain and spinal cord early in spontaneous disease
At the onset of clinical disease, lesions are present in the cerebellum that shows apoptosis and necrosis, loss of neutropil, some edema and extensive myelin loss. Prominent glial cell responses are present, suggesting that the lesions have been ongoing for some time even in young mice (Fig. 6A–E). Moderate lesions occur near the hippocampus/thalamus with ventricular involvement, as well as some scattered gray matter lesions in the mid- brain (Fig. 6F, G). Demyelination occurs in areas surrounding sites of inflammation (Fig. 6D, E). In older WT BG1 mice, and Rag−/− BG1 mice that have converted to chronic disease, CNS inflammation is associated with effacement of nervous tissue, leaving stromal elements and dense accumulations, principally comprised of lymphocytes (Fig. 6H–K). Essentially no white matter lesions are seen within the cerebral cortex. Mice that developed paralytic disease have diffuse meningeal, submeningeal and central canal lymphocyte infiltration along most of the length of the spinal cord that is more severe in the caudal region. In this area, infiltration occurs in the neuronal tissue and rarefied tissue is observed (Fig. 6L). Consistent with minimal BG1 T cell signaling in peripheral organs (Fig. 3G, H), no histological damage is observed outside of the CNS.
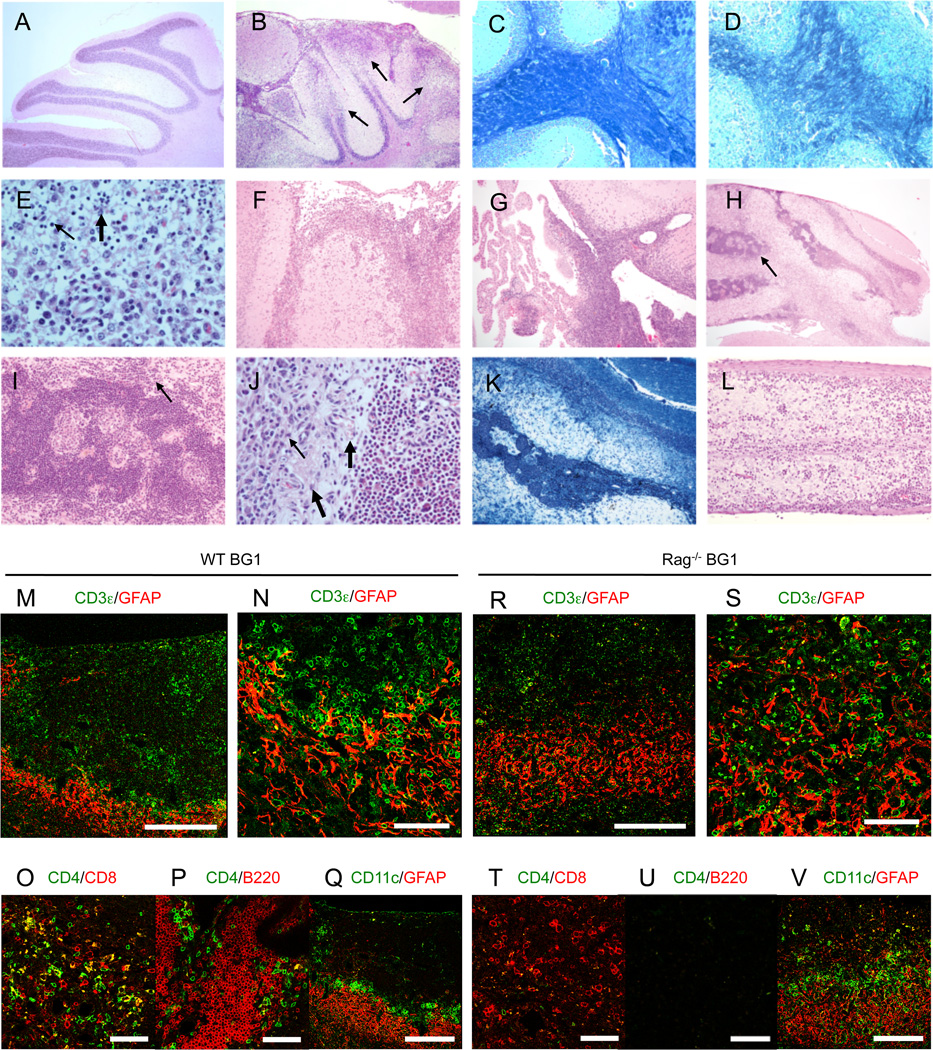
BG1 mice develop lesions within the gray and white matter of the brain and spinal cord. (A) H&E stain of cerebellum of a healthy C57BL/6 mouse. (B) Earlier phases of disease in BG1 mice are characterized by involvement of broad areas of cerebellar meninges and gray and white matter with necrosis and apoptosis accompanied by a dense glial and inflammatory response (thin arrows). (C) Luxol Fast Blue stain of healthy and (D) diseased area of the cerebellum of a BG1 mouse shows that the inflammatory response is intermixed with necrotic and apoptotic cells and results in loss of myelin (loss of dark blue stain) along with other elements of the neuropil. (E) CNS lesions show focal extensive regions of necrosis and apoptosis accompanied by mixed inflammatory cell populations. The inflammatory cellular component can vary within the site but is typically composed of mixtures of neutrophils (thick arrow), lymphocytes (thin arrow) and microglial elements. At this stage it is common to find extension of the inflammatory response from (F) colliculus meninges and (G) choroid plexus into adjacent gray matter nervous tissue. In late stages of disease, (H, I, J) the cerebellum and other affected sites become distinguished by the presence of dense accumulations of lymphocytes (H, thin arrows) and the replacement of nervous tissue elements by a vascular rich stroma (thick arrows) containing scattered inflammatory cells (thin arrows in I, J). (K) The extensive loss of myelin within the cerebellum is visualized by a lack of Luxol Fast Blue stain. (L) Lesions of the spinal cord are less dramatic but consistent with the pattern where meningeal and perivascular accumulations of predominately lymphocytes extend into the adjacent neuropil. Images are representative examples of six WT BG1 and Rag−/− BG1 mice with early onset or late spontaneous CNS disease. (M–Q) Diseased WT BG1 and (R–V) Rag−/− BG1 mice develop T cell accumulations surrounded and intermixed with GFAP+ astrocytes. Lymphocyte aggregates in WT BG1 Tg mice contain (M) CD8, CD4 T cells and (P) B cells, and are (Q) ringed with CD11c+ cells. The yellow stained cells in (O) are not T cells, as no CD4+ CD8+ double positive T cells are observed in BG1 mice, and likely represents either non-specific binding or staining of cellular debris (R–V) Lesions in diseased Rag−/− BG1 mice contain (R–U) CD8 T cells, without CD4 T cells or B cells. Lesions are ringed with (V) CD11c+ and GFAP+ astrocytes. Images are representative of three WT BG1 and four Rag−/− BG1 mice analyzed. Scale bar; 250µm on A, E, F and J, 60µm on the others.
To elucidate the cellular organization of immune cells within the brain of WT BG1 and Rag−/− BG1 mice, the cerebella of diseased mice were stained with cell-specific antibodies. In both WT BG1 and Rag−/− BG1 mice, T cells were found within the lesions in the CNS (Fig. 6M–V). Most of the T cells were detected adjacently to GFAP-positive astrocytes or around the vessels (Fig. 6M, N, R and S). While CD8 T cells are the dominant lymphocyte population in the cerebellum of Rag−/− BG1 mice, lesions in WT BG1 mice contain CD4 T cells and B cells as well (Fig. 6 O, P, T and U). In the cerebellum of both WT BG1 and Rag−/− BG1 mice, CD11c-positive APCs are found densely located at the lesion edge suggesting that these lesions remain in a pathologically active state (Fig. 6Q, V).
B cells regulate spontaneous relapsing-remitting disease course in BG1 mice
Differences in the frequency and severity of spontaneous disease between WT BG1 and Rag−/− BG1 mice suggested that CD8 T cell extrinsic mechanisms contribute to the regulation of CD8 T cell-mediated CNS disease. Rag−/− BG1 mice, which show a greater susceptibility to spontaneous CNS disease than WT BG1 mice, lack B cells and non-transgenic T cells. To identify whether B cell or CD4 T cell populations contribute to the regulation of CD8 T cell-mediated CNS autoimmunity, we generated MHC II−/− (IAbβ−/−) BG1 and B cell−/− µMT−/−) BG1 mice. We observed that µMT−/− BG1 mice are highly susceptible to spontaneous CNS disease, displaying clinical symptoms at a higher rate than littermate heterozygous controls (Fig. 7, Table 1). In addition to the increase in frequency, 83% of the µMT−/− BG1 mice developed chronic clinical disease, as compared to the relapsing-remitting disease most often observed in WT BG1 and Rag−/− BG1 mice (Fig. 7C). MHC II−/− BG1 showed a mild increase in frequency of spontaneous CNS disease as compared to littermate heterozygous controls (Fig. 7 D, Table 1). The increase in clinical symptom frequency, severity and changes in disease course in µMT−/− BG1 occurs even though heterozygous littermate controls have similar numbers of CD8 T cells that target the CNS (Fig. 7 E–J). These data indicate that B cells can contribute to the regulation of CD8 T cell-mediated CNS autoimmunity.
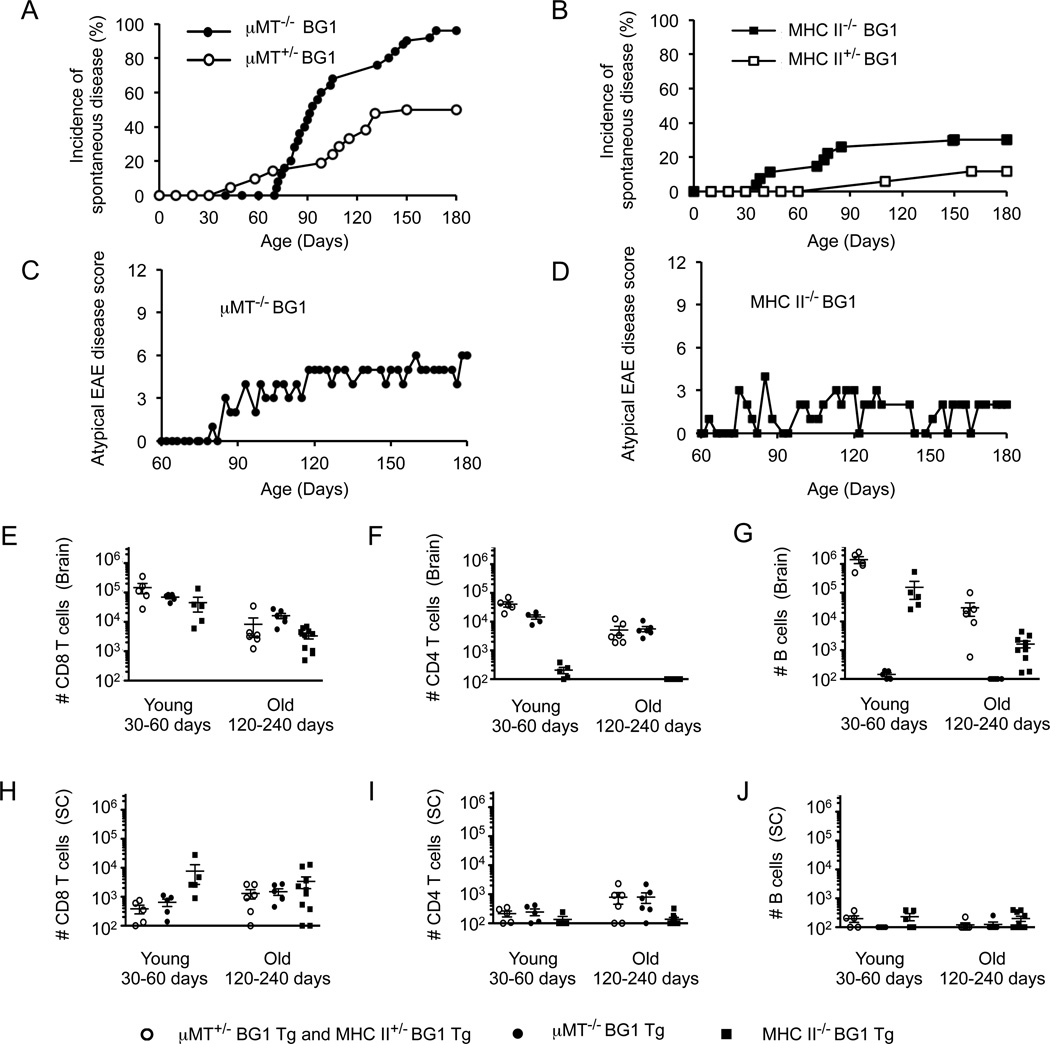
B cell-deficient BG1 mice are highly susceptible to chronic atypical EAE-like disease. Incidence of first clinical signs of EAE-like symptoms in (A) µMT−/− BG1 (n = 25) or µMT+/− BG1 (n = 21) and (B) MHC II−/− BG1 (n = 27) or MHC II+/− BG1 (n = 17). (C) Representative examples of clinical disease in µMT−/− BG1 and (D) MHC II−/− BG1 mice. (E–J) Absolute numbers of lymphocytes in the (E–G) brain or (H–J) spinal cord were determined. Each cell population was identified as shown in Fig. 4. Results are from at least five individual mice. Error bars represent SEM.
Virally activated BG1 CD8 T cells induce CNS autoimmunity with pathological features different from mice with spontaneous disease
The prolonged clinical disease course, along with T cell entry into the CNS parenchyma and associated inflammatory response observed in BG1 mice, is clinically and histologically distinct from the disease pathologies induced by activated MBP-specific CD8 T cells. Activated MBP-specific CD8 T cells induce a rapid clinical disease that targets the white matter throughout the brain, forming perivascular cuffs composed of lymphocytes, macrophages, as well as a few neutrophils. These perivascular cuffs are associated with necrosis and apoptosis of surrounding neuronal tissue and demyelination (24). The global differences in the CNS disease pathologies created by MBP- versus GFAP-specific CD8 T cells could be a result of the T cells targeting different antigens, or a product of how disease was induced (spontaneous versus active induction). To test whether activated BG1 CD8 T cells target the CNS and induce CNS disease that resembles the spontaneous disease in BG1 mice or disease created by activated MBP-specific CD8 T cells, BG1 CD8 T cells were transferred into C57BL/6 recipient mice, and recipient mice infected with Vac:GFAP.
We observed that Vac:GFAP infection of C57BL/6 mice carrying a small population of BG1 CD8 T cells developed severe ataxia and lethargy within 7 days, clinical symptoms highly similar to those induced by activated MBP-specific CD8 T cells (video S2). No mice displayed evidence of ascending paralysis. The severity of the disease was commensurate with the number of BG1 CD8 T cells transferred into the C57BL/6 recipient mice. C57BL/6 mice that received 5 × 105 CD8 T cells or greater succumbed to morbidity, whereas mice receiving 1.25 × 105 or 2.5 × 105 CD8 T cells developed an acute disease that dissipated after a week (Fig. 8A). No onset of disease was observed in mice infected with Vac:GFAP which did not receive BG1 CD8 T cells, when recipient mice were Gfap−/− or when recipient mice were infected with Vac:Neg virus. During the peak of Vac:GFAP-induced CNS disease, BG1 CD8 T cells within the CNS were strongly biased towards KLGR1+ short-term TEFF and TEM cells and expressed high levels of IFNγ, GZB and IRF-4 (Fig. 8C, D, E and S2).
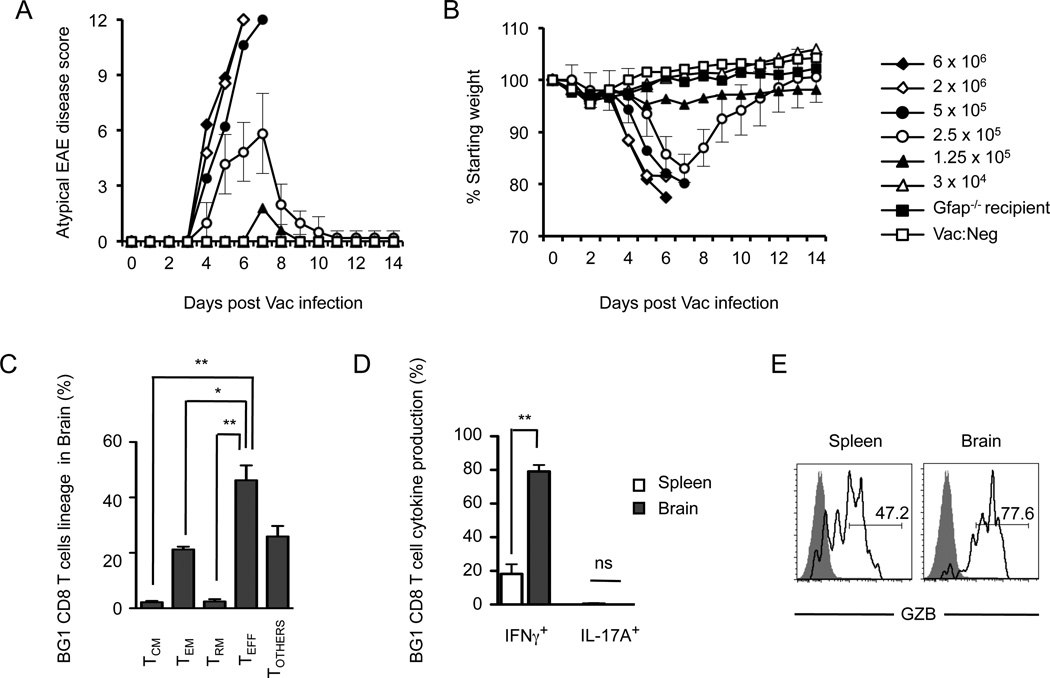
Viral activation of the naïve BG1 CD8 T cell induces CNS infiltration and autoimmunity. Vac:GFAP infection of C57BL/6 mice carrying small populations of BG1 CD8 T cells induces (A) atypical EAE disease symptoms and (B) weight loss. Each group consisted of 7–9 mice. For clarity, Standard Deviation error bars are shown only for mice receiving 2.5 × 105 BG1 CD8+ cells. (C) Identification of brain-resident BG1 CD8 T cell subsets following Vac:GFAP infection (see Fig. S1). (D) BG1 CD8 T cells present within the brain following Vac:GFAP activation are strong producers of IFNγ, and express (E) granzyme B. Phenotypic results are derived from or are a representative example of at least four individual mice. Error bars represent SEM. *; P=<0.05, **; P=<0.01, ns; Not Significant.
Within the brain of mice with virally triggered disease, affected areas are widely disseminated, and include gray and white matter of the brainstem, midbrain and cerebellum (Fig. 9). Vac:GFAP-activated BG1 CD8 T cells also targeted the cerebral cortex, a site not affected during spontaneous CNS disease in BG1 mice. Within the brain, infiltrating lymphocytes localized to the meninges and vascular/perivascular space, with minimal parenchymal infiltration. Surrounding the perivascular and meningeal infiltrates, moderate acute necrosis and apoptosis is observed with some demyelination and neuronal cell loss, without a significant parenchymal inflammatory cell response. Commensurate with the lack of ascending paralytic disease, infiltration within the spinal cord is scant to very mild and almost exclusively meningeal. T cell infiltrates were not observed in organs other than the CNS (not shown).
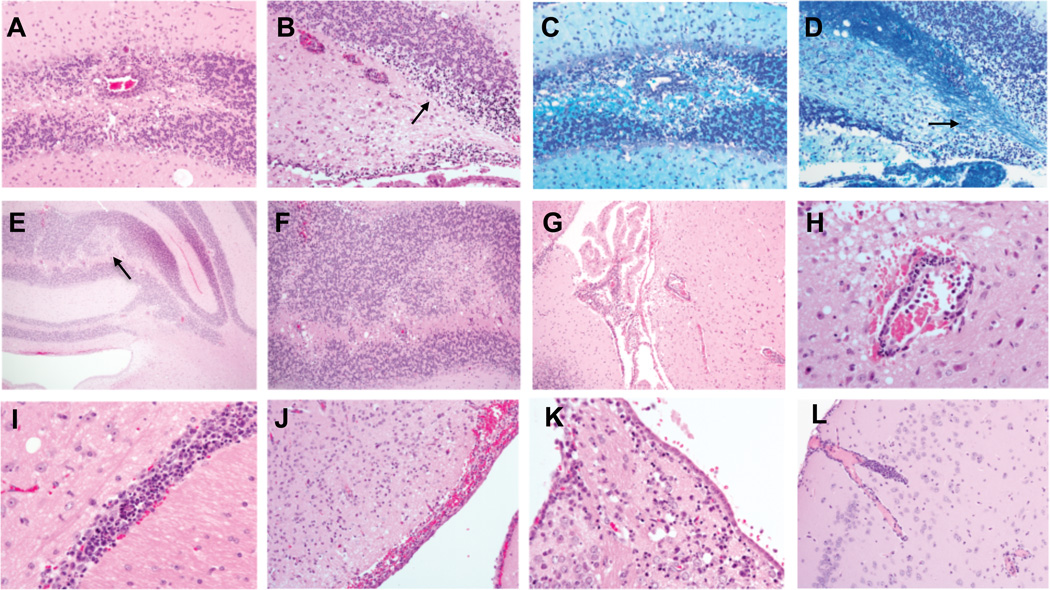
Vac:GFAP activated BG1 CD8 T cells that target the CNS are typically confined to meningeal, perivascular or other localized sites within the gray and white matter of brain. (A, B) Targeting of the cerebellum causes lymphocytic perivascular cuffing and focal extensive areas of cellular apoptosis and necrosis of the granular cell layer (arrow), but only mild inflammatory cell accumulation. (D, E) In targeted area some associated demyelination (loss of LFB stain) occurs. (C, F) In animals that survive acute disease loss of neurons are observed (pale area, arrow in C) along with the presence of widely cuffed vessels, vacuolated white matter and glial cells but only scant parenchymal lymphocytes. (G) Lymphocytic inflammation within the choroid plexus and (H) nearby vessels (surrounded by apoptotic bodies and lymphocytes) do not extend into the adjacent nervous tissues. (I, J) Diffuse meningitis and perivasculitis including (K) a region of the habenular nucleus shows scattered apopotosis and individual cell necrosis with a mild, mixed inflammatory cell accumulation. (L) Vessels within the cerebral cortex are occasionally marginated and cuffed with lymphocytes (some of which are apoptotic within adjacent parenchyma) but extension into the surrounding nervous tissue is minimal. Images are representative examples of six mice with Vac:GFAP induced CNS disease.
These data demonstrate that Vac:GFAP activation of BG1 CD8 T cells generated primarily classical TEFF and TEM CD8 T cells that targeted the brain and induced a rapid, acute and clinically severe CNS autoimmunity. Many of the histological features and clinical symptoms of this CNS autoimmunity are similar to in vitro activated MBP-specific CD8 T cell-induced disease, and stand in contrast to the spontaneous relapsing- remitting disease that developed in BG1 mice.
Discussion
The experiments described here demonstrate that CD8 T cells that target a protein expressed in astrocytes can avoid tolerance mechanisms prior to entering into the CNS and causing autoimmune damage. GFAP-specific CD8 T cells were observed to target both the gray matter and white matter of the brain and spinal cord. Furthermore, the composition and location of the lesions, as well as the clinical symptoms of disease, were dependent upon the triggering event that activated the CNS-reactive CD8 T cells. These findings indicate that CD8 T cells which target astrocytes can induce CNS autoimmune disease, and strongly suggest that different CD8 T cell effector lineages contribute to heterogeneity in CNS lesion formation and clinical disease course.
To contribute to the CNS autoimmune disease process, self-reactive CD8 T cells have to avoid thymic and peripheral tolerance mechanisms and when activated, target the CNS. Similar to several myelin proteins, a splice variant of GFAP is expressed within the thymus (48, 52). This thymic expression, however, appears to be below the detection of developing BG1 thymocytes as negative selection was not observed in BG1 mice that express GFAP, even though BG1 CD8 T cells recognize target cells loaded with nanomolar concentrations of GFAP264–272 peptide. Demonstrating that GFAP-specific CD8 T cells target the CNS is in contrast to mice that express the neo-antigen HA under the GFAP promoter, which when challenged with HA-specific CD8 T cells either develop immune tolerance or enterocolitis (53, 54), but not CNS autoimmunity. The HA-specific CL4 T cell was originally isolated from a non-HA expressing mouse infected with influenza. Thus, it is possible that the HA-specific CL4-TCR is more sensitive to the viral neo-antigen than the BG1 CD8 T cells are to endogenous GFAP. Consistent with the hypothesis, mice that doubly express the CL4 TCR and GFAP-HA die by 6 days of age, a rapid death that may occur prior to the ability of significant numbers of CD8 T cell to migrate to the CNS(53). Furthermore, when naïve CL4 CD8 T cells are adoptively transferred into GFAP-HA mice, a strong proliferative responses ensues directed at the HA neo-antigen, a phenotype that we do not observe in BG1 adoptive transfers (54) (see Figure 3). Supporting the hypothesis that astrocyte specific T cells can escape tolerance induction and drive CNS autoimmunity, S100β-specific CD4 T cells (which also target astrocytes) can develop and the immunization of Lewis rats with S100β peptides induces CNS autoimmunity with gray and white matter pathology (55, 56). Thus, when activated, both CD4 and CD8 T cells that target astrocytes can contribute to CNS autoimmunity.
The observation that BG1 CD8 T cells are largely ignorant of endogenous GFAP protein is similar to studies analyzing a human TCR specific for an epitope of PLP, and in contrast to studies of CD8 T cells in C3H mice that target MBP. PLP-specific CD8 T cells were found to develop in mice expressing HLA-A3 and remained primarily ignorant of PLP, with few mice succumbing to spontaneous CNS disease. When activated, these PLP-specific CD8 T cells induce CNS autoimmunity, indicating that the PLP epitope is presented within the CNS (27). MBP-specific CD8 T cells in contrast, have been found to be subject to tolerance induction, with some pathogenic MBP-specific T cell clonotypes using a variety of strategies to avoid thymic and peripheral tolerance mechanisms including stealing the MBP epitope from APCs, and expressing two TCRα chains (57, 58). CD8 T cells specific for MOG have been shown to induce CNS disease indicating that these T cells develop, however the phenotype of these T cells prior to activation has not been determined. In addition, CD8 T cells specific for multiple different myelin epitopes have been identified in both MS patients and healthy controls, indicating that CD8 T cell tolerance to CNS proteins in incomplete. Thus, similar to CD4 T cell responses targeting CNS antigens (6, 38–41), the thymic expression of CNS proteins does not fully purge pathogenic CD8 T cells from the peripheral T cell repertoire.
MS has traditionally been thought to be an autoimmune disease of the white matter of the CNS. However, more recent studies have identified gray matter lesions in MS patients that appear at the earliest stages and accumulate over time (2, 59–62). The observation that CD8 T cells are present within gray matter lesions of MS patients suggests that CD8 T cells reactive to antigens other than myelin proteins may contribute to MS disease progression (2, 62). Astrocytes reside within the white and gray matter of the CNS. GFAP, an intermediate filament protein, is a prototypical astrocyte-specific antigen that is expressed throughout the gray matter and white matter of the brain and spinal cord (63). Normally, astrocytes express low levels of MHC class I, which increases during inflammation (64). In MS lesions, for example, the expression level of GFAP increases and peptides derived from GFAP are presented by MHC class I and class II molecules (65–67). Data presented here indicate that GFAP-specific CD8 T cells can target the CNS and induce gray matter and white matter pathology, inducing apoptosis and necrosis of neuropil and extensive myelin loss. Although GFAP-specific T cells isolated from MS patients have not been studied, GFAP-specific CD8 T cells have been isolated from patients with type 1 diabetes, indicating autoimmune-prone individuals carry T cells with this reactivity pattern (68). In addition, CD8 T cells that target astrocytes and neurons have also been suggested in Rasmussen encephalitis cells (69).
The clinical signs of MS are highly variable and often include symptoms of upper motor neuron disease such as hyperreflexia, ataxia, spasticity and visual defects. In some cases there is evidence of lower motor neuron disease such as sensory defects and partial or complete paralysis. In the majority of patients these symptoms manifests as a relapsing-remitting disease, usually converting over time to a chronic progressive stage (1, 4, 8). The idea that different T cell lineages associate with unique aspects of CNS disease is well documented for encephalogenic CD4 T cells. The detailed analysis of CD4 T cells responding to several different neuroantigens has shown that the effector lineage and activation status of CD4 T cells within the CNS regulates disease location, severity and clinical outcome (70–72).
Our genetic studies suggest that CD4 T cells and B cells have complex roles in regulating CD8 T cell-initiated CNS autoimmunity. In contrast to the relapsing/remitting disease course observed in WT BG1 and Rag−/− BG1 mice, B cell−/− BG1 mice succumbed to a chronic disease course. This fundamental change in the manifestation of spontaneous CNS disease indicate that B cells can limit CD8 T cell-induced CNS autoimmunity, and promote or allow for disease remissions. These data also suggest that CD4 T cells, absent in Rag−/− BG1 mice while present in B cell−/− BG1 mice, may promote CD8 T cell-induced CNS autoimmunity, possibly by providing CD8 T cell help. The elimination of B cells, resulting in enhanced CNS autoimmunity may seem counter-intuitive considering that Rituximab, B cell-depletion therapy, shows promise in treating MS patients with relapsing/remitting disease (73). However, studies of CD4 T cell-mediated EAE models have shown that B cells can impact CNS autoimmunity in multiple ways, detrimentally through the production of CNS-specific antibodies and acting as antigen presenting cells for pathogenic CD4 T cell, as well as in a suppressive manner by producing anti-inflammatory cytokines (73–79). Because B cells do not efficiently cross-present antigens on MHC I molecules to CD8 T cells (80), B cell may play primarily a suppressive role in CD8 T cell-initiated CNS disease.
How different CD8 T cell effector and memory lineages impact the MS disease process is poorly understood. Within the CSF and CNS of MS patients, CD8 T cells can express IFNγ and/or IL-17, and have TCM or TEM phenotypes (71–75)(81–85). The population of TRM phenotype CD8 T cells has not been studied in MS patients. In the experiments presented here, we observed that GFAP-specific CD8 T cells induce distinct CNS disease pathologies and a clinical disease course depending upon how they are activated. BG1 CD8 T cells responding to a viral infection generate a population of highly cytotoxic effector CD8 T cells that rapidly enter into the CNS and localize to the meninges and vascular/perivascular space within both gray and white matter, causing localized necrosis and apoptosis. Clinically, these mice succumb to acute ataxia, spasticity and lethargy. In contrast to this acute disease, BG1 T cells that are spontaneously recruited to the CNS and cause relapsing- remitting disease show a diversity of effector lineages. These primarily include lymphocytes that phenotypically resemble TRM and other poorly defined subsets of CD8 T cells, with a paucity of GZB- or IFNγ- expressing TEFF-, TEM- or TCM- lineage CD8 T cells. The memory-phenotype CD8 T cells that do develop enter into the CNS parenchyma as well as localize to the meninges and perivascular space, and induce significant inflammatory responses. Why these different CD8 T cell subsets localize differently and how CD8 T cells that do not make conventional inflammatory cytokines induce CNS disease is unclear. In models of CD4-EAE, the expression of IFNγ can limit the ability of CD4 T cells to enter into the parenchyma and affect T cell trafficking patterns (86). In addition, the extensive inflammatory response during spontaneous CNS disease suggest that BG1 T cells can directly on indirectly induce NF-ΚB signaling in glial cells that may propagate the inflammatory response (87, 88).
In summary, the experiments described here indicate that CD8 T cells that target non-myelin CNS antigens can avoid tolerance mechanisms, and drive unique aspects of inflammatory CNS autoimmunity, including the targeting of gray matter and white matter of the brain. The composition and location of the lesions, as well as clinical symptoms created during spontaneous disease versus a viral induction, strongly suggests that the triggering event that activates auto-reactive CD8 T cells contributes to heterogeneity in CNS disease and clinical disease course.
Acknowledgements
The authors thank Drs. Brian Stadinski for helpful discussions and Rebecca Smith for technical assistance with some of the experiments.
This work was supported by Beckman Young Investigator Award and National Institutes of Health (NIH) Grants RAI088495A and DK095077 (to E.S.H.). K.S. was supported by a fellowship from the Japanese Society for the Promotion of Science. E.S.H. is a member of the University of Massachusetts Medical School Diabetes and Endocrinology Research Center (NIH Grant DK32520).
References
Full text links
Read article at publisher's site: https://doi.org/10.4049/jimmunol.1302911
Read article for free, from open access legal sources, via Unpaywall:
https://www.jimmunol.org/content/jimmunol/192/7/3029.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Unveiling GFAP Astrocytopathy: Insights from Case Studies and a Comprehensive Review of the Literature.
Antibodies (Basel), 13(4):79, 25 Sep 2024
Cited by: 0 articles | PMID: 39449321 | PMCID: PMC11503365
Review Free full text in Europe PMC
Single-cell RNA sequencing reveals the pro-inflammatory roles of liver-resident Th1-like cells in primary biliary cholangitis.
Nat Commun, 15(1):8690, 07 Oct 2024
Cited by: 0 articles | PMID: 39375367 | PMCID: PMC11458754
Glial fibrillary acidic protein astrocytopathy presented as meningitis: A case report.
Heliyon, 10(5):e26827, 22 Feb 2024
Cited by: 0 articles | PMID: 38434407 | PMCID: PMC10907785
CD8+ Tissue-Resident Memory T Cells: Versatile Guardians of the Tissue.
J Immunol, 212(3):361-368, 01 Feb 2024
Cited by: 2 articles | PMID: 38227907
IL-21, not IL-17A, exacerbates murine primary biliary cholangitis.
Clin Exp Immunol, 215(2):137-147, 01 Feb 2024
Cited by: 1 article | PMID: 37708215
Go to all (61) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Gp130-dependent astrocytic survival is critical for the control of autoimmune central nervous system inflammation.
J Immunol, 186(11):6521-6531, 22 Apr 2011
Cited by: 77 articles | PMID: 21515788
A pathogenic role for myelin-specific CD8(+) T cells in a model for multiple sclerosis.
J Exp Med, 194(5):669-676, 01 Sep 2001
Cited by: 377 articles | PMID: 11535634 | PMCID: PMC2195947
Viral infection triggers central nervous system autoimmunity via activation of CD8+ T cells expressing dual TCRs.
Nat Immunol, 11(7):628-634, 06 Jun 2010
Cited by: 107 articles | PMID: 20526343 | PMCID: PMC2900379
Role of T cell-glial cell interactions in creating and amplifying central nervous system inflammation and multiple sclerosis disease symptoms.
Front Cell Neurosci, 9:295, 05 Aug 2015
Cited by: 16 articles | PMID: 26300731 | PMCID: PMC4525059
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NIAID NIH HHS (2)
Grant ID: T32 AI007349
Grant ID: R21 AI088495
NIDDK NIH HHS (4)
Grant ID: R01 DK095077
Grant ID: P30 DK032520
Grant ID: DK32520
Grant ID: DK095077
PHS HHS (1)
Grant ID: RAI088495A





