Abstract
Free full text

COVID‐19, nausea, and vomiting
Abstract
Exclusion of nausea (N) and vomiting (V) from detailed consideration as symptoms of COVID‐19 is surprising as N can be an early presenting symptom. We examined the incidence of NV during infection before defining potential mechanisms. We estimate that the overall incidence of nausea (median 10.5%), although variable, is comparable with diarrhea. Poor definition of N, confusion with appetite loss, and reporting of N and/or V as a single entity may contribute to reporting variability and likely underestimation. We propose that emetic mechanisms are activated by mediators released from the intestinal epithelium by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) modulate vagal afferents projecting to the brainstem and after entry into the blood, activate the area postrema (AP) also implicated in anorexia. The receptor for spike protein of SARS‐CoV‐2, angiotensin 2 converting enzyme (ACE2), and transmembrane protease serine (for viral entry) is expressed in upper gastrointestinal (GI) enterocytes, ACE2 is expressed on enteroendocrine cells (EECs), and SARS‐CoV‐2 infects enterocytes but not EECs (studies needed with native EECs). The resultant virus‐induced release of epithelial mediators due to exocytosis, inflammation, and apoptosis provides the peripheral and central emetic drives. Additionally, data from SARS‐CoV‐2 show an increase in plasma angiotensin II (consequent on SARS‐CoV‐2/ACE2 interaction), a centrally (AP) acting emetic, providing a further potential mechanism in COVID‐19. Viral invasion of the dorsal brainstem is also a possibility but more likely in delayed onset symptoms. Overall, greater attention must be given to nausea as an early symptom of COVID‐19 and for the insights provided into the GI effects of SARS‐CoV‐2.
Introduction
In addition to the prominent respiratory and pyrexic symptoms of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), 1 the symptom complex can include symptoms associated with the digestive tract including anorexia, nausea, vomiting and diarrhea. Of these, diarrhea has received the most attention in the original literature, 2 , 3 , 4 , 5 , 6 commentaries, 7 , 8 , 9 and reviews. 10 , 11 , 12 Surprisingly, nausea and vomiting have not been discussed in detail. This is a serious omission as nausea and vomiting can be the presenting symptoms, as exemplified by the first cases of COVID‐19 in the United States 13 and China. 2 Initially, the occurrence of nausea and vomiting was reported to be “uncommon” with an incidence of < 4%, 7 but more recently, their prevalence has been described as “common.” 11 Superficially at least, the incidence of nausea and vomiting seems comparable with that for diarrhea (~ 5–6% in some systematic studies 14 , 15 ); ~ 10% in others 10 ; some studies report incidences for diarrhea of ≥ 30%. 3 , 11
It is important to understand the incidence of nausea and vomiting during COVID‐19 and the mechanisms by which this can occur. Patients with digestive tract symptoms, including nausea, vomiting, and diarrhea, may have delayed hospital admission and a worse clinical outcome than those without. 16 It has been argued 3 that the value of diarrhea as a clinical symptom in the diagnosis and spread of COVID‐19 may be underestimated, particularly as digestive tract symptoms may precede respiratory ones. 11 However, nausea is an acute onset, early warning of a problem in the upper digestive tract (primarily stomach and small intestine), which can receive air when swallowing, and is a component of the body's epithelial defenses, triggered in the “natural” world by ingested toxins. 17 This mean that nausea may be the first indication of gastrointestinal (GI) infection by SARS‐CoV‐2; interestingly, “virus” is the Latin word for “poison.” Given the current paucity of knowledge, we first address key questions about COVID‐19 in relation to the prevalence of nausea and vomiting and then discuss plausible mechanisms.
How good is the epidemiological data on the incidence of nausea and vomiting in COVID‐19?
Nausea is a self‐reported sensation, but unless it is clearly defined for the patient, particularly in epidemiological studies and drug trials, there is a potential for confusion. Firstly, reporting of the symptom needs to be specifically requested to avoid the practice of merging the terms nausea, retching, and vomiting into a single adverse event. Secondly, the patient will base their understanding of the word on what they have been told nausea feels like (most likely originally by a parent or a clinician) or their previous experience of nausea (most likely in motion sickness); this may also be influenced by cultural and linguistic differences. Thirdly, symptoms that can occur with nausea or independently such as lack of desire to eat, dyspepsia, and epigastric discomfort short of overt pain may or may not be reported as nausea. These problems are compounded by the lack of a validated diagnostic biomarker for nausea although elevated plasma vasopressin and gastric dysrhythmia are associated with nausea. 18 , 19
It has been proposed to use pictograms to improve digestive tract symptom assessment, 20 helping to minimize linguistic and translation issues—particularly important when assessing comparability of symptom reporting from a global pandemic where cultural perceptions and understanding of nausea may complicate accurate reporting.
The literature contains multiple definitions of nausea (table 1.1, 18 for 30 definitions from the literature), which may be associated with different body areas (e.g. head, chest, and epigastrium). 19 Here, we define nausea as “a self‐reported, unpleasant sensation associated with the desire to vomit or the feeling that vomiting is imminent”. 18 Although vomiting is easier to define (the forceful oral expulsion of upper digestive tract contents), it may be identified incorrectly and used interchangeably with retching (unproductive, forceful, externally visible, rhythmic contractions of thoracic, and abdominal muscles usually preceding vomiting 21 ) and can be confused with intense gastroesophageal reflux. Nausea and vomiting can be reported as a single entity (nausea or vomiting, 1 nausea and/or vomiting 22 ) although they are a sensation and motor act respectively.
However, in reviewing the publications where data on the incidence of both nausea and vomiting have been collected, it is clear that both can be an early “acute” symptom of COVID‐19. Detailed data need to be collected on the incidence of N & V at all stages of SARS‐CoV‐2 infection. We highlight four important issues related to the epidemiology.
- 1.
Nausea and vomiting need to be reported separately. In the studies where this has been performed, the incidences are either identical (e.g. 1% vs 1% 1 ; 5% vs 5% 23 ; 3.7% vs 3.7% 24 ) or higher than for vomiting (e.g. 10.1% vs 3.6%; 17.3% vs 5.0%; 26.4% vs 15.4% 25 , 26 , 27 A similar equivalence in the incidence of nausea and vomiting is seen for SARS and MERS where the overall incidences of both are higher at ~ 20%. 12 Although the data on COVID‐19 are limited, the incidence of nausea appears to be highly variable with a range of 1–30% 23 , 26 (Figure 1). Our literature analysis of 41 studies of >12,000 COVID‐19 patients (including children 28 ) in which nausea and/or vomiting data was reported, showed that the median incidence of nausea was 10.5% and that of diarrhea reported in the same studies was 11%; the latter comparable with the prevalence of 10.4% reported in a recent systematic review 58 but higher than in some other studies. 14 , 15 These findings support the view that nausea and diarrhea should be given equal prominence as symptoms. Notably, the median incidence of vomiting was 7% (Fig. 1), an incidence comparable with the 6.9% reported for abdominal pain in a systematic review/meta‐analysis. 58 It should be noted that as far as we are aware, separate detailed data on the incidence of nausea and vomiting in male and female patients have not been published and apart from the studies in children, there is no detailed age‐stratified symptom data.
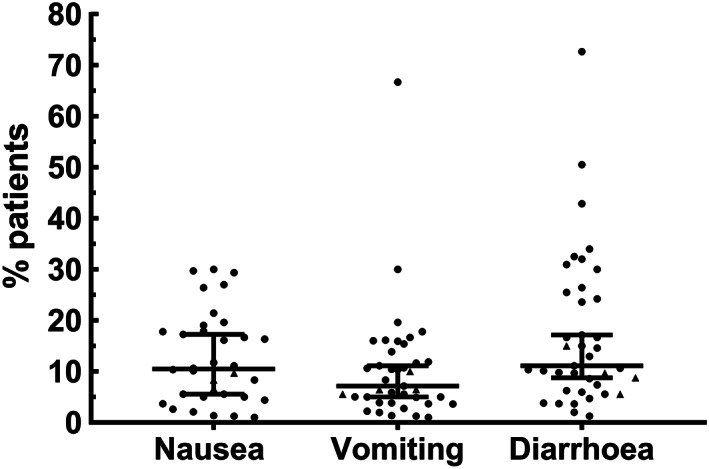
The incidence of nausea and/or vomiting and diarrhea from 41 clinical studies 1 , 2 , 4 , 5 , 16 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 : nausea, n = 34; vomiting, n = 39; diarrhea, n = 39. Values for individual studies are shown together with the median and 95% confidence intervals. No distinction was made between nausea and vomiting in 10 studies, so the same incidence was used in both categories. The total number of patients in the studies was 12 239. Five studies (data indicated by triangles) reported only data on children (1.5 months to 17 years). 28 , 34 , 37 , 38 , 51
- 2.
What is the co‐occurrence of nausea and vomiting with other digestive tract symptoms in COVID‐19? Considering the likely effects of SARS‐CoV‐2 on the digestive tract (discussed further), a relationship between symptoms such as nausea/vomiting and diarrhea would not be unexpected but identifying the time of onset of each postinfection is essential to assessing their relative relevance for diagnosis. Without data on individual patients, it is not possible to draw firm conclusions, but some studies have begun to report the incidence of single and groups of symptoms. For example, nausea or vomiting with diarrhea has been reported with an incidence of 4.9% 29 and 4.3%. 22 In the 41 clinical studies analyzed above, 27 reported the incidence of nausea/vomiting and diarrhea with our analysis showing a correlation (Spearman R, 0.8 and 0.7). Identification of the temporal expression of symptoms and their clustering will be essential for classification of the ways in which SARS‐CoV‐2 infection can present, and to quickly recognize evolving patterns of presentation, as this may be a reflection of viral load and its relative impact on the respiratory and digestive tracts, and subsequently the brain (see further for the discussion). One study noted that patients with nausea and vomiting, and diarrhea were more likely to have fever, than those with either digestive symptom alone. 30 Finally, it is recognized that type II diabetes is a risk factor for COVID‐19. 59 The frequent occurrence of gastroparesis in diabetic patients 60 may make this group either more sensitive to the nausea and vomiting during SARS‐CoV‐2 infection or result in more intense symptoms.
- 3.
What is the severity of the nausea? To date, the published studies have only reported on the presence or not of nausea and vomiting, with no descriptions of their severity, comparable with those used routinely to assess these symptoms as side‐effects of anticancer chemotherapy. 61 A study of almost 2000 patients reported that GI symptoms (predominantly diarrhea, nausea, vomiting, and abdominal pain) were “judged to be mild” in 74% of patients, 31 which could imply it was more severe in the remaining 26%. Additionally, there is a paucity of data on the temporal pattern of nausea and vomiting during the course of the disease. One publication commented that digestive symptoms (including vomiting but not nausea) became more pronounced as the severity of disease increased. 16 In another, the prevalence of nausea or vomiting was twice as high in hospitalized patients (not intensive care unit) compared with the emergency department (10% [6.2–15.8] vs 22.6% [19.5–26.1]) but interestingly reduced to 10.2% (6.9–14.7%) in patients in intensive care. 32 A recent commentary on the research priorities in the digestive manifestations of COVID‐19 62 called for “a more rigorous and systematic assessment of the prevalence, spectrum and severity of digestive manifestations”. We agree but strongly argue that nausea should be included as part of the symptom cluster. Similarly, the utility of mobile technology for collecting real‐time data on symptoms of COVID‐19 (see further) has been demonstrated, 63 , 64 but the symptom check list included in the supporting information 63 included diarrhea, abdominal pain and skipping meals, not nausea or vomiting. Such mobile technology is ideally suited to obtain data on the presence and intensity of nausea with a high temporal resolution and to investigate changing patterns of symptom expression.
- 4.
The relationship between nausea and appetite. A few studies report lack/loss of appetite or poor/low appetite, with an incidence apparently higher than that of nausea (23.2%, 49.5%, and 40%). 16 , 22 , 30 Of these studies, some 16 , 30 did not report nausea but others 22 reported “nausea and/or vomiting” with an incidence of 12% but the percentage of patients with either nausea or vomiting or both was not reported. While reduced appetite is a common symptom of infection, it can also be secondary to nausea as indicated by a preclinical study of the cytokine growth differentiation factor 15 65 demonstrating anorexia by induction of nausea and/or engaging with emetic mechanisms; this is also the case in cancer chemotherapy. 61 The incidence of anorexia in COVID‐19 patients in intensive care units is reported to be ~ 2× that of patients who were not. 33 As described above, it is likely that some patents will report loss of appetite when in reality, they have nausea, emphasizing the need for clarity and consistency of definitions when collecting data on nausea (as proposed for diarrhoea 3 ). The recent study of real‐time tracking of self‐reported symptoms 63 identified “skipped meals” as the symptom with the second highest odds ratio of SARS‐CoV‐2 infection after loss of smell; nausea as a potential underlying cause of skipping meals should be investigated further. Although children become infected with COVID‐19, they appear to be relatively asymptomatic in relation to adults, but there are reports of vomiting. 28 , 34 However, young children may not be capable of verbalizing nausea and food refusal in children has been argued to be an indicator of nausea. 66
In summary, more attention should be given to recognizing nausea, which as we highlighted above must be clearly defined to avoid confusion, as a potential early symptom of COVID‐19 particularly when collecting epidemiological data. It is plausible that infected subjects who do not have the classical symptoms (fever and persistent cough and loss of taste/smell) may have initially experienced mild, transient nausea, and dismissed it. Accordingly, asymptomatic but SARS‐CoV‐2 positive individuals, many of whom are likely to be in the younger age groups, need to be investigated to determine if they have recently had an episode of nausea, which was dismissed or ascribed to “food poisoning.” Thus, because nausea is an early alerting symptom of a challenge (toxic food and chemicals, bacterial toxin, and virus) to the upper digestive tract, this may be an overlooked initial symptom of SARS‐CoV‐2 infection. Such studies are also important because if nausea is an early symptom of COVID‐19, the vomiting that may follow would lead to ejection of aerosolized, viral contaminated vomit, as occurs in patients infected with norovirus. 67
Finally, it is important to recognize that subjects with nausea (primarily studied in motion sickness) may have a reduction in cutaneous blood flow to the forehead manifest as “pallor” 68 associated with “cold sweating.” 18 As the forehead is a common site at which temperature is taken, nausea induced by SARS‐CoV‐2 could be a factor confounding detection of raised core temperature by a single time point measurement of forehead temperature alone.
How could SARS‐CoV‐2 induce nausea and vomiting?
There are no formal studies at present so we have reviewed the effects of SARS‐CoV‐2 (and other coronaviruses) on the digestive tract in the light of knowledge of the established mechanisms of nausea and vomiting; this is the same approach that has been used to understand the pathogenesis of other symptoms (e.g. diarrhoea 10 ).
SARS‐CoV‐2 can readily access the digestive tract by several routes (Fig. 2). We hypothesize that SARS‐CoV‐2 would induce acute (first few days postinfection) nausea and vomiting by causing the release of key hormones from the enteroendocrine cells (EECs) in the mucosa of the upper GI tract or after gaining direct entry into the blood, by acting directly within the brainstem.
- 1.
Pathways of nausea and vomiting
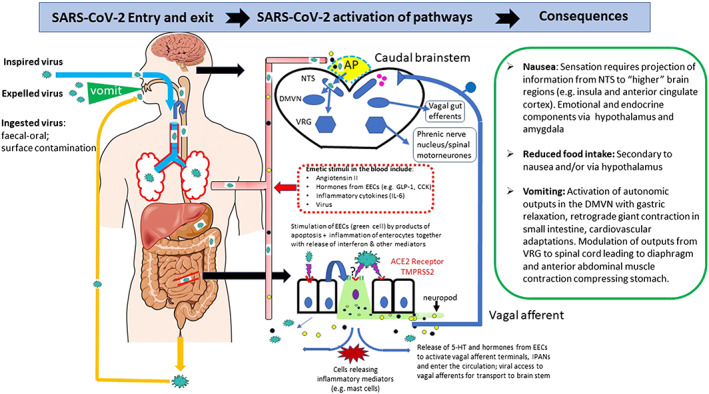
Diagram summarizing the potential mechanisms by which SARS‐CoV‐2 could induce nausea and vomiting. The left‐hand panel shows the routes by which virus can enter the body to access the airways and digestive tract. Air can enter the digestive tract during swallowing. The presence of high levels of angiotensin converting enzyme 2 receptor in the airways and digestive tract is indicated by red lines. The middle panel shows the potential mechanisms (based on evidence discussed in the text) for the interaction of the virus with the digestive tract epithelium leading to release of neuroactive agents from enteroendocrine cells and inflammatory mediations which act either by stimulating/sensitizing abdominal vagal afferent terminals and/or act on the area postrema in the dorsal medulla where the blood brain and blood cerebrospinal fluid barriers are relatively permeable. In addition, angiotensin II levels may increase and have central actions together with virus, which may enter the circulation from damaged lungs and digestive tract epithelia. The right hand panel summarizes the consequences of vagal afferent and area postrema activation to induce nausea and vomiting by projection of information to higher brain regions (nausea and anorexia) and vomiting by motor pathways in the ventral brainstem and spinal cord. 5‐HT, 5‐hydroxytryptamine; ACE 2, angiotensin converting enzyme receptor 2; AP, area postrema; CCK, cholecystokinin; DMVN, dorsal motor vagal nucleus; EEC, enteroendocrine cells in the digestive tract; GLP‐1, glucagon‐like peptide‐1; IL‐6, interleukin‐6; NTS, nucleus tractus solitarius; TMPRSS2, transmembrane protease serine 2; VRG, ventral respiratory group of neurones. [Color figure can be viewed at wileyonlinelibrary.com]
Induction of nausea and vomiting from the digestive tract occurs via two main mechanisms 18 , 69 :
- a)
Activation of abdominal vagal afferents, either mechanoreceptors located in the muscle layers or afferents terminating in close apposition to the mucosa activated by neuroactive agents (e.g. 5‐hydroxytrytamine [5‐HT], substance P [SP], and cholecystokinin [CCK]) released locally from the EECs to act at receptors expressed by afferent nerve terminals. Among the EEC cells, the enterochromaffin (EC) cells in the duodenum and jejunum contain most of the 5‐HT present in the body of humans and other mammals, which when released by chemical (e.g. cancer chemotherapy and oral emetics) and pathological (e.g. inflammation) stimuli, has a particularly prominent role in induction of emesis via activating abdominal vagal afferent nerve terminals 70 (Fig. 2)
- b)
Release of neuroactive agents into the hepatic portal vein and subsequently the systemic circulation to act on the area postrema, a circumventricular organ located at the caudal extremity of the fourth ventricle where the blood–brain and cerebrospinal fluid‐brain barriers are relatively permeable (Fig. 2). These agents may originate from the EECs (e.g. glucagon like‐1 peptide and CCK) but also as cytokines from inflamed or damaged GI epithelia.
Both the vagal afferents and area postrema efferents activate the nucleus tractus solitarius (NTS), subjacent to the area postrema in the brainstem, which in turn activates the visceral and somatic motor pathways for vomiting elsewhere within the brain stem and also sends projections to higher brain regions generating the sensation of nausea 18 , 19 (Fig. 2). The release of neuroactive agents from EECs is implicated in both acute, early onset nausea and vomiting when the release is by Ca++‐mediated exocytotic mechanisms and in more protracted responses when inflammatory‐like responses and cell damage are implicated. 61 The likelihood that nausea requires less intense activation of the above pathways than does vomiting means that greater emphasis should be given to nausea rather than vomiting as an initial symptom of digestive tract infection by SARS‐CoV‐2.
- 2.
ACE2 in the digestive tract, viral entry, nausea, and vomiting by peripheral actions of SARS‐CoV‐2
Release of neuroactive agents from the digestive tract acting locally (vagal afferents) and systemically (area postrema) has been implicated in nausea and vomiting induced by toxins ingested with food, therapeutic drugs, and bacteria. 69 , 71 , 72 , 73 We know of only one study investigating a direct link between viral infection and the mechanisms of nausea and vomiting. The virus commonly responsible for infantile gastroenteritis (vomiting and diarrhea are symptoms), rotavirus, has been shown to infect and replicate in EC cells (from midgut carcinoid tumor and GOT1 cells), increasing intracellular [Ca++] and secretion of 5‐HT from the EC cells. Notably, 5‐HT secretion increased by 6 h after infection. 74 Using supernatant from viral infected MA104 cells, secretion of 5‐HT from GOT1 and primary EC tumor cells occurred within 60 min demonstrating that it was most likely due to viral protein(s) from infected cells rather than due to cell replication. Cell [Ca++] was elevated and 5‐HT secreted in response to the enterotoxic glycoprotein NSP4 previously shown to be secreted from human intestinal cells infected with rotavirus. In mice infected with rotavirus, there was colocalization between viral proteins and 5‐HT‐containing EC cells, and Fos immunohistochemistry showed intense activation of the NTS suggestive of vagal afferent activation (see above). The same laboratory also demonstrated an ability of adenovirus 41, a cause of acute gastroenteritis with diarrhea and vomiting, to increase 5‐HT release from human EC cells. 75
Finally, it is worth noting that rotavirus infected infant mice displayed diarrhea, which was treated with the 5‐HT3 receptor antagonist ondansetron, which also improved weight gain and interestingly, attenuated viral shedding. 76 The latter may be of clinical value as ondansetron and other 5‐HT3 antagonists are widely available for treatment of acute cancer chemotherapy induced nausea and vomiting. 69
So, what of SARS‐CoV‐2? The angiotensin converting enzyme II (ACE2) protein is the receptor for the spike protein of SARS‐CoV‐2, providing a key component of the entry route for the virus into cells as is the case for SARS and MERS. 77 , 78 Cellular serine protease transmembrane protease serine‐2 (TMPRSS2) is required together with ACE2 for viral entry. 79 , 80 Early human and rodent studies identified ACE2 in the digestive tract and particularly not only the ileum but also other regions of the small intestine. 77 , 81 , 82 More recent studies have confirmed the presence of ACE2 in human small intestine, including the duodenum, and some have shown expression of TMPRSS2. 3 , 83 , 84 , 85 In particular, the studies have identified ACE2 and TMPRSS2 in “absorptive enterocytes” and more generally “epithelial cells.” 6 , 84 Expression of ACE2 mRNA has also been shown in the relatively sparse EECs within the enterocytes of human stomach and ileum, 86 although a transcriptomic and proteomic study of human and mouse intestinal EECs did not highlight ACE2 as among the most highly expressed receptors in either species. 87 These data find some concordance with a study of mouse small intestine appearing to indicate that although the ACE2 receptor was highly expressed in enterocytes, there was no expression in “endocrine” cells (although the viral receptor HCoV‐229E (ANPEP) was present in this population). 3 However, mice lack the ability to vomit and other differences between human and rodent digestive tract suggest caution should be exercised in translating from one species to another. 88 , 89 A recent study using human small intestinal organoids cultured from adult stem cells in a variety of different media showed infection of enterocytes by SARS‐COV and SARS‐CoV‐2. 83 Notably, however, EECs stained with an antibody for Chromogranin A+ (CGA) were never infected; CGA is expressed in all EEC cells, particularly the monoamine containing subpopulation. 90 These data contrast with the expression of ACE2 by EECs within human stomach and ileum and with an ability of enteroviruses to infect EECs. 86 , 91 They suggest that the SARS viruses are unlikely to enter EECs to cause GI disturbances by directly stimulating the release of hormones from these cells. However, key experiments remain. For example, might the EECs of the organoid differ in some way from the native EEC, preventing viral entry? Nevertheless, if confirmed, it seems that the induction of enterocyte apoptosis and the rapidly increasing inflammatory and immune cell milieu surrounding the EECs (measured as increased mRNA for cytokines and interferon stimulated genes) 83 will secondarily induce the release of hormones from the embedded EECs (Worthington et al 92 for mechanisms).
In addition, gut microbiota have been implicated in modulation of gut inflammation 93 but as yet, it is unclear how changes in gut microbiota during SARS‐CoV‐2 infection may contribute to the generation of symptoms. For example, a pilot study of the fecal microbiome in hospitalized patients showed an association between changes in the microbiota, fecal levels of SARS‐CoV‐2 and the severity of respiratory symptoms. 94 However, only one of the patients reported GI symptoms (diarrhea before admission) and none during admission. Microbiota were included in a model of SARS‐CoV‐2‐induced diarrhoea. 10 This is of particular relevance as ACE2 has been implicated in the regulation of the gut microbiome and susceptibility to colitis. 95
Finally, we should not overlook the impact of viral‐induced digestive tract damage, loss of integrity, and release of an “inflammatory soup” on infection, resulting in sensitization of vagal afferents via receptors for diverse agents including cytokines, prostaglandins, amines, and purines. There is limited mechanistic data on cytokine‐induced emesis. One study in the cat showed that intramuscular injections of IL‐2‐induced emesis after the area postrema was ablated, providing indirect evidence for a peripheral action, 96 but in rat area postrema cells IL‐1β increased Ca++ influx. 97 A more recent study of the cytokine growth differentiation factor 15 favors a central action. 65 Activation of prostanoid receptors on vagal nerve terminals represents an additional pathway shown to induce vomiting. 98
An observation of potential therapeutic relevance comes from a study with the NK1 receptor antagonist aprepitant in patients with HIV. 99 NK1 antagonists are used to treat cancer chemotherapy‐induced emesis by both central and peripheral actions with interactions between the 5‐HT3 and the NK1 receptor. 61 , 69 In HIV patients, aprepitant administered over 2 weeks reduced plasma concentrations of cytokines (G‐CSF, IL‐6, IL‐8, and TNFα) with in vitro and preclinical studies in nonhuman primates providing additional evidence for antiretroviral and anti‐inflammatory effects. Overall, this combination of actions of aprepitant together with the anti‐emetic activity 69 may make the drug worthy of investigation in COVID‐19 patients particularly as aerosolized vomit is a viral vector. 67
Further studies are required to investigate the presence of ACE2 and TMPRSS2 in the various subpopulations of EEC cells (particularly EC cells). 100 , 101 Additionally, it should be noted that many of the GI hormones capable of inducing nausea and vomiting can also reduce appetite and food intake (e.g. CCK and glucagon like‐1 peptide) by acting on the area postrema and hypothalamic circumventricular organs, so may also relate to the appetite suppression reported on admission in a few studies, but at a relatively high incidence. 16 , 30
Finally, although attention has focused on the intestinal epithelium, the early human studies reported the presence of ACE2 in the smooth muscle cells of the muscularis mucosa and muscularis propria and the vascular smooth muscle and endothelium in the stomach, small intestine, and colon. 77 This does not necessarily mean that SARS‐CoV‐2 gains entry into the muscle cells as additional binding to a chemokine coreceptor may be needed. Nevertheless, further studies are needed to assess whether actions of SARS‐CoV‐2 on GI smooth muscle could be involved in the pathophysiology of GI symptoms. The presence of ACE2 (and TMPRSS2) in these locations requires study using current molecular techniques and functional studies using in vitro pharmacological models 102 to assess whether actions of SARS‐CoV‐2 on GI smooth muscle could be involved in symptom generation. For example, changes in GI motility may be involved in COVID‐19 diarrhea, described more as an increase in stool frequency rather than liquidity 16 so may not be (completely) of epithelial origin.
- 3.
ACE2 in the gut‐brain axis, viral entry, nausea, and vomiting by central actions of SARS‐CoV‐2
As the COVID‐19 pandemic has progressed, there has been increasing awareness of the central nervous system as a target, with some authors including nausea, vomiting, and headache as “neurologic signs.” 103 There is preclinical data showing ACE2 expression in the brain notably in the NTS and the area postrema 104 and more limited evidence for the presence of ACE2 in the brain. 105 , 106 The view that SARS‐CoV‐2 can access the brain via the blood (the circumventricular organs where the blood–brain barrier is relatively permeable being assumed to be particularly vulnerable) or by neuroinvasion via the vagal afferents (which supply both the lungs and the digestive tract) is growing in strength 105 , 107 , 108 ; SARS‐CoV is neuroinvasive and has been detected in human brain. 109 While the vagal transport of SARS‐CoV‐2 is unlikely to account for acute onset of nausea and vomiting, access of the virus to the NTS and more directly via the area postrema is a potential mechanism by which later nausea and vomiting may be induced and a pathway by which regulation of breathing and blood pressure can be compromised. Vomiting is a key symptom of porcine hemagglutinating encephalomyelitis coronavirus, which can access the brain stem, but it must be noted that it has not been determined if this effect is due to a central (e.g. NTS) or peripheral effect. 110
Viral damage to the dorsal brain stem, which has a major role in regulation of digestive tract function, could contribute to the development of postviral digestive tract symptoms (e.g. gastroparesis), which may emerge during recovery.
Are there lessons to be learnt for the digestive tract from the interactions between SARS‐CoV and ACE2 in the respiratory system?
The interactions between SARS‐CoV, ACE2, and the respiratory epithelium in the pathogenesis of acute respiratory distress syndrome (ARDS)/acute lung injury, has a number of lessons for understanding the effects of SARS‐CoV‐2 on the digestive tract (for reviews see. 111 , 112 The key observations in the airways, mostly from mice studies are (i) ACE2 is protective against acute lung injury by its conversion of angiotensin II (AII) to angiotensin (1–7) and angiotensin I to angiotensin (1–9), with severe ARDS in Ace2 KOs being improved by administration of an angiotensin 1 receptor (AT1) antagonist; (ii) Once initiated the severity of ARDS is influenced by ACE2 and other components of the renin‐angiotensin system (RAS); and (iii) Ace2 KOs are resistant to SARS‐CoV; in wild‐type mice, SARS‐CoV downregulates ACE2 in the lungs with consequent elevation of AII levels, also reported in patients with ARDS. 113 These studies give insights into viral effects on the lungs, but as the studies were in mice which do not vomit, 74 , 75 the effect of ACE2 KO on vomiting is unknown.
The presence of ACE2 and SARS‐CoV‐2 in digestive tract epithelium and the superficial similarities between airways and the digestive tract, including the presence of EECs linked to the vagus, 114 enables speculation on the effects of ACE2 and SARS‐CoV‐2 interactions in the digestive tract in relation to nausea and vomiting.
- a)
SARS‐CoV‐2 infection in the digestive tract causes inflammatory damage, with consequences for induction of nausea and vomiting, discussed above. The inflammatory effects are local and systemic. In human ARDS plasma IL‐6, increases and interestingly treatment with recombinant human ACE2 showed a trend to decrease IL‐6, 113 an effect if repeated in COVID‐19 patients may be beneficial to the digestive tract as well as any effects of cytokines on nausea, vomiting, and food intake.
- b)
Interaction between SARS‐CoV‐2 and ACE2 in both the respiratory and digestive tracts will elevate plasma AII concentrations; in patients, the average concentration of plasma AII is ~ 3× higher than in healthy subjects with an average concentration of ~ 300 pg/mL. 35 In preclinical studies, AII has been shown to induce emesis when given intravenously and into the cerebral ventricles 115 , 116 and to increase neuronal activity in the area postrema 117 , 118 , 119 with AT1 receptors implicated. This provides a plausible mechanism by which elevated plasma AII in COVID‐19 could induce nausea and vomiting, providing an additional trigger to those from the GI tract (Fig. 2). There is also preclinical evidence that systemic AII can have central effects to suppress respiration, which are not dependent upon the integrity of the AP. 120 Circulating AII can also affect gastric and intestinal motility and could contribute the genesis of nausea (and diarrhea). The EC50 for contraction of the human small intestine in vitro was 1.5 × 10−8 M, 121 a concentration less than the average of ~ 300 pg/mL reported in COVID‐19 patients, 35 which equates to ~3 × 10−7 M, and an earlier study reported contraction of gastric muscle with a threshold concentration of AII of 10−9 M. 122
- c)
Angiotensin 1 and 2 receptors are found throughout the digestive tract and modulate smooth muscle activity as well as epithelial secretion and absorption. 123 Actions of elevated AII could influence any of these functions with motility changes most likely to be implicated in nausea and abdominal pain (these could also involve local vasoconstriction) and both motility and epithelial transport in pathogenesis of diarrhea. Understanding the digestive tract effects of SARS‐CoV‐2 will require a detailed understanding of the RAS in the human digestive tract.
Possible role of medications and coexisting morbidities in nausea and vomiting in COVID‐19 patients
The above sections have focused on the direct effects of SARS‐CoV‐2 on the digestive tract as the most likely cause of nausea and vomiting. However, two other potential causes need to be recognized briefly for completeness. Firstly, prior to presentation at hospital, patients may be prescribed medications which may have nausea and vomiting as side effects. Of particular relevance are ACE inhibitors for treatment of hypertension (e.g. lisinopril) and antidiabetic drugs (e.g. metformin and liraglutide). Hospitalized patients being treated for COVID‐19 may be treated with cytokine modulators, antibiotics, and antiviral agents, which may have nausea as a side‐effect and should be considered when assessing the incidence of nausea. One publication reported that the incidence of nausea and vomiting did not differ whether patients did or did not receive either antibiotics or antiviral treatments. 4 Secondly, nausea and vomiting may be secondary to damage outside the upper digestive tract. Although the lungs and airways might seem an obvious site given particularly considering their vagal afferent innervation, there is no evidence that nausea or vomiting can be induced from the airways. However, there is preclinical evidence that activation of respiratory system vagal afferents can block induction of vomiting. 124 Renal failure is also a symptom of severe COVID‐19 and in other settings nausea and vomiting can be symptoms with the mechanism not defined, although it is unlikely to be due to renal afferents. 125 The liver is also damaged in severe COVID‐19, and although it has a vagal afferent supply projecting to the brainstem, 126 there is no evidence implicating the afferents directly in nausea and vomiting. 127 It is likely that nausea and vomiting associated with hepatic failure is due to loss of the detoxifying function of the liver. Cardiac failure also occurs in severe cases of COVID‐19. Ventricular cardiac afferents in the cat have been shown to be capable of inducing vomiting and are thought to be responsible for the nausea and vomiting associated with a myocardial infarct 128 so could provide a pathway for their induction in cardiac failure.
Therapeutic opportunities
Optimal anti‐emetic treatment relies on an understanding of the pharmacology of the pathways involved. On the basis of our hypothesized mechanisms described above, we would expect the combination of a 5‐hydroytryptamine3 (e.g. ondansetron and palonosetron) and a neurokinin1 receptor antagonist (e.g. aprepitant) to be effective as they would block at the peripheral site of activation/sensitization of vagal afferents and central sites in the dorsal brain stem (NTS/area postrema). 69 It is interesting to note that the synthetic corticosteroid dexamethasone is being used to treat the inflammatory actions effects of SARS‐CoV‐2, but the use of dexamethasone as an anti‐emetic may not be widely known outside the oncology community. In the treatment of the intense nausea and vomiting associated with some anticancer chemotherapy regimens, dexamethasone has been used for 40 years 129 in conjunction with more classical anti‐emetics (e.g. metoclopramide, ondansetron, and aprepitant) 61 , 69 where it enhances their efficacy with a particular improvement in nausea (see other studies 61 , 69 , 130 for references). The anti‐emetic effect of dexamethasone has been ascribed to its anti‐inflammatory effect on the intestinal epithelium as its efficacy is greater in the delayed phase of chemotherapy‐induced emesis where inflammatory damage releasing systemic mediators acting on the area postrema has been implicated. 69 However, there has been little formal research on the mechanism(s) underlying the anti‐emetic effects of dexamethasone, but such studies may provide additional insights into its effects on the digestive tract in COVID‐19.
The provision of anti‐emetics to patients presenting with nausea will help alleviate this unpleasant symptom, reduce the probability that the patient will vomit, but if vomiting is present they will reduce it and the associated potential viral spread by aerosolization in vomit.
Concluding comments
This review has highlighted nausea and vomiting as acute symptoms of COVID‐19 with a focus on incidence of occurrence (Fig. 1) and on mechanisms (Fig. 2). The literature we reviewed shows the incidence of nausea ranges from 1% to 30% but with a median incidence of 10.5%, similar to that of diarrhea but less than that of vomiting (7%). A systematic review and meta‐analysis of the incidence of nausea and vomiting as separate symptoms in COVID‐19 is needed. However, many studies do not report nausea, and although more studies report vomiting, it is not clear if this represents difference in data collection methodology. We argue that there should be a greater focus on collecting data on nausea as it may be a more sensitive early marker of digestive tract infection (i.e. prior to fever and cough and probably preceding diarrhea) in addition to representing a neglected symptom with a particular potential to be overlooked in children. The absence of diagnostic biomarkers for nausea is an additional challenge. 130 A possible mechanism is discussed whereby nausea and vomiting can be initiated by SARS‐CoV‐2 within the blood acting directly on brainstem structures. We also propose that the EECs may be a target for SARS‐CoV‐2 responsible (in part) for the pathogenesis of nausea and vomiting; these proposals require further testing using available techniques and may give insights into supportive treatments (e.g. 5‐HT3 and NK1 receptor antagonists). Further studies of mechanisms are needed and in addition to in vitro studies of EEC cell lines these may include studies in the ferret, which is sensitive to SARS‐CoV‐2 131 and which is an established animal model for the study of emetic mechanisms and anti‐emetic pharmacology. 132 The incidence of nausea/vomiting and diarrhea in COVID‐19 is lower than in SARS, but the SARS‐CoV‐2 protein spike is 10–20× more potent at binding ACE2 compared with SARS, 133 so a comparison of the effects on different cell populations in the GI epithelium may provide an insight into the reasons. The brain stem as a site at which mediators from the digestive tract induce nausea and vomiting and loss of appetite is discussed. Finally, as the majority of patients who develop COVID‐19 recover, the potential development of postinfection functional bowel disorders 134 needs to be monitored, where nausea and vomiting may also be symptoms. Thus, the recognition of nausea and vomiting as key symptoms should lead to greater understanding of how SARS‐CoV‐2 attacks the GI tract and brainstem so preventative measures can treat the symptoms and limit the spread of the virus by vomiting.
Acknowledgments
We thank Dr Heidi Shuk Han Ng, LASEC and SBS, The Chinese University of Hong Kong, Shatin, New Territories, Hong Kong for comments on the final drafts of the original submission.
Notes
Andrews, P. L. R. , Cai, W. , Rudd, J. A. , and Sanger, G. J. (2021) COVID‐19, nausea, and vomiting. Journal of Gastroenterology and Hepatology, 36: 646–656. 10.1111/jgh.15261. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
Declaration of conflict of interest: GJS and JAR receive research funding from Takeda Pharmaceuticals. The other authors declare no conflict of interest.
Authors contribution: PLRA wrote the first draft, and all authors modified drafts and provided intellectual input.
Financial support: No funding directly attributed to this article.
References
Full text links
Read article at publisher's site: https://doi.org/10.1111/jgh.15261
Read article for free, from open access legal sources, via Unpaywall:
https://onlinelibrary.wiley.com/doi/pdfdirect/10.1111/jgh.15261
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1111/jgh.15261
Article citations
Migraine inhibitor olcegepant reduces weight loss and IL-6 release in SARS-CoV-2-infected older mice with neurological signs.
J Virol, 98(7):e0006624, 30 May 2024
Cited by: 2 articles | PMID: 38814068
Migraine and gasdermin D: a new perspective on the inflammatory basis of migraine.
Acta Neurol Belg, 124(3):981-986, 25 Mar 2024
Cited by: 0 articles | PMID: 38526645
Trend and Co-occurrence Network of COVID-19 Symptoms From Large-Scale Social Media Data: Infoveillance Study.
J Med Internet Res, 25:e45419, 14 Mar 2023
Cited by: 2 articles | PMID: 36812402 | PMCID: PMC10131634
A qualitative study of Covid-19 effects on nutrition associated problems in recovered patients.
BMC Nutr, 9(1):29, 13 Feb 2023
Cited by: 0 articles | PMID: 36782270 | PMCID: PMC9924848
Digestive system infection by SARS‑CoV‑2: Entry mechanism, clinical symptoms and expression of major receptors (Review).
Int J Mol Med, 51(3):19, 20 Jan 2023
Cited by: 1 article | PMID: 36660939 | PMCID: PMC9911086
Review Free full text in Europe PMC
Go to all (31) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The roles of nausea and vomiting in COVID-19: did we miss something?
J Microbiol Immunol Infect, 54(4):541-546, 17 Oct 2020
Cited by: 12 articles | PMID: 34435559 | PMCID: PMC7568482
Review Free full text in Europe PMC
Expression of ACE2, TMPRSS2, and SARS-CoV-2 nucleocapsid protein in gastrointestinal tissues from COVID-19 patients and association with gastrointestinal symptoms.
Am J Med Sci, 366(6):430-437, 01 Sep 2023
Cited by: 1 article | PMID: 37660993
Clinical Characteristics of 195 Cases of COVID-19 with Gastrointestinal Symptoms COVID-19 with Gastrointestinal Symptoms.
Turk J Gastroenterol, 32(2):148-154, 01 Feb 2021
Cited by: 4 articles | PMID: 33960938 | PMCID: PMC8975350
SARS-CoV-2-mediated encephalitis: Role of AT2R receptors in the blood-brain barrier.
Med Hypotheses, 144:110213, 26 Aug 2020
Cited by: 15 articles | PMID: 33254519 | PMCID: PMC7449115
Funding
Funders who supported this work.
Medical Research Council (1)
Proposal to develop a gastrointestinal (GI) neuropharmacology platform within the Neurogastroenterology Group (NGG)
Professor Gareth John Sanger, Queen Mary University of London
Grant ID: G0900805

 1
1




