Abstract
Background
Tuberculosis (TB) and indoor air pollution (IAP) are equally critical public health issues in the developing world. Mongolia is experiencing the double burden of TB and IAP due to solid fuel combustion. However, no study has assessed the relationship between household solid fuel use and TB in Mongolia. The present study aimed to assess the association between household solid fuel use and TB based on data from the Mongolian National Tuberculosis Prevalence Survey (MNTP Survey).Method
The MNTP Survey was a nationally representative population-based cross-sectional survey targeting households in Mongolia from 2014 to 2015, with the aim of evaluating the prevalence of TB. The survey adopted a multistage cluster sampling design in accordance with the World Health Organization prevalence survey guidelines. Clusters with at least 500 residents were selected by random sampling. A sample size of 98 clusters with 54,100 participants was estimated to be required for the survey, and 41,450 participants were included in the final analysis of the present study. A structured questionnaire was used to collect information on environmental and individual factors related to TB. Physical examination, chest X-ray, and sputum examinations were also performed to diagnose TB.Results
The use of solid fuels for heating (adjusted odds ratio (aOR): 1.5; 95% confidence interval (CI): 1.1-2.1), male gender (aOR: 2.2; 95% CI: 1.6-3.2), divorced or widowed (aOR: 2.6; 95% CI: 1.7-3.8), daily smoker (aOR: 1.8; 95% CI: 1.3-2.5), contact with an active TB case (aOR: 1.7; 95% CI: 1.2-2.3), being underweight (aOR: 3.7; 95% CI: 2.4-5.7), and previous history of TB (aOR: 4.3; 95% CI: 3.0-6.1) were significantly associated with bacteriologically confirmed TB after adjusting for confounding variables.Conclusion
The use of solid fuels for heating was significantly associated with active TB in Mongolian adults. Increased public awareness is needed on the use of household solid fuels, a source of IAP.Free full text

Association between household solid fuel use and tuberculosis: cross-sectional data from the Mongolian National Tuberculosis Prevalence Survey
Abstract
Background
Tuberculosis (TB) and indoor air pollution (IAP) are equally critical public health issues in the developing world. Mongolia is experiencing the double burden of TB and IAP due to solid fuel combustion. However, no study has assessed the relationship between household solid fuel use and TB in Mongolia. The present study aimed to assess the association between household solid fuel use and TB based on data from the Mongolian National Tuberculosis Prevalence Survey (MNTP Survey).
Method
The MNTP Survey was a nationally representative population-based cross-sectional survey targeting households in Mongolia from 2014 to 2015, with the aim of evaluating the prevalence of TB. The survey adopted a multistage cluster sampling design in accordance with the World Health Organization prevalence survey guidelines. Clusters with at least 500 residents were selected by random sampling. A sample size of 98 clusters with 54,100 participants was estimated to be required for the survey, and 41,450 participants were included in the final analysis of the present study. A structured questionnaire was used to collect information on environmental and individual factors related to TB. Physical examination, chest X-ray, and sputum examinations were also performed to diagnose TB.
Results
The use of solid fuels for heating (adjusted odds ratio (aOR): 1.5; 95% confidence interval (CI): 1.1–2.1), male gender (aOR: 2.2; 95% CI: 1.6–3.2), divorced or widowed (aOR: 2.6; 95% CI: 1.7–3.8), daily smoker (aOR: 1.8; 95% CI: 1.3–2.5), contact with an active TB case (aOR: 1.7; 95% CI: 1.2–2.3), being underweight (aOR: 3.7; 95% CI: 2.4–5.7), and previous history of TB (aOR: 4.3; 95% CI: 3.0–6.1) were significantly associated with bacteriologically confirmed TB after adjusting for confounding variables.
Conclusion
The use of solid fuels for heating was significantly associated with active TB in Mongolian adults. Increased public awareness is needed on the use of household solid fuels, a source of IAP.
Background
Tuberculosis (TB) is a major public health issue and is one of the leading 10 global causes of death, particularly in low and middle-income countries [1]. According to the World Health Organization (WHO), a majority of the 10 million incident TB cases and 1.4 million TB deaths occurred in low and middle-income countries in 2019 [2]. Mongolia is one of the high TB burden countries in the Western Pacific region, with a TB prevalence of 428 per 100,000 population [3]. Five to 15% of individuals with mycobacterium TB infection develop active TB, particularly when they have risk factors such as an immunosuppressive condition (HIV, diabetes, age, malnutrition), deteriorating socioeconomic conditions, environmental exposure, and behavioral factors (smoking and alcohol consumption) [4]. Indoor air pollution (IAP) from the use of solid fuels (e.g., coal) is a potential risk factor for TB, given the negative impact it has on the airway defense mechanism [5]. A majority of health-related exposure to air pollution from solid fuels occurs around the household in low and middle-income countries [6].
Over 3 billion people continue to rely on household solid fuels (e.g., wood, coal, crop residue, animal dung, and charcoal) and use simple stoves for cooking and heating [7]. According to the 2010 household census in Mongolia, 45.2% of households lived in traditional ghers, 29.5% lived in houses equipped with simple stoves, and 72% used solid fuels in everyday cooking and/or heating [8]. In developing countries, household combustion of solid fuels emits health-damaging pollutants, causing a high level of IAP [9]. Burning solid fuels with inefficient stoves or open hearths produces various pollutants, including particulate matter (PM), methane, carbon monoxide, polyaromatic hydrocarbons, and volatile organic compounds [10, 11]. Exposure to such compounds indoors is likely to have a greater impact on health than exposure to the same compounds outdoors. In fact, around 4.3 million people die globally due to household IAP every year [10]. During the cold season in Mongolia, households burn over 600,000 tons of coal for domestic heating, and 80% of air pollution in the city is caused by household solid fuel combustion [12]. Air pollution is attributed to 9.7% of all deaths in Ulaanbaatar (UB) City, Mongolia’s capital [13].
Many studies have reported a direct relationship between the exposure to household solid fuels and negative health consequences, including chronic obstructive pulmonary disease, acute lower respiratory infection, lung cancer, cardiovascular disease, and cataracts [11, 14, 15]. However, the relationship between household IAP from solid fuel use and active TB remains controversial. A case-control study in India reported a significant positive association between biomass fuel use and pulmonary TB [16]. Yet, other studies did not find strong evidence for a positive association between household solid fuel use and TB [17, 18]. While a systematic review in 2013 reported strong evidence for an association between IAP and TB [19], a more recent systematic review of IAP and TB concluded the level of association between the two to be very low [18]. Another systematic review of solid fuel use and active TB concluded that the risk of active TB is dependent on the type of fuel used, with the highest risk being associated with biomass burning [20]. Regarding other types of fuel, the number of published studies on this topic was small, and the results in some studies did not account for confounding factors [20].
TB is one of the leading causes of mortality from respiratory disease in Mongolia [21], and IAP from solid fuel use is also a major issue [13, 22]. However, there is no evidence supporting a relationship between solid fuel use and active TB in the country. Against this backdrop, the present study aimed to assess the association between household solid fuel use and active TB based on data from the Mongolian National Tuberculosis Prevalence Survey (MNTP Survey), a large-scale study of a representative Mongolian adult population.
Methods
Country
Mongolia is divided administratively into 21 provinces and the capital, UB City. Almost half of the population lives in UB City, with 250 people per square kilometer, while provinces have about 2 people per square kilometer [8]. Each province has a rural area and a provincial center. The rural area consists of small rural administrative units called “soum,” and the provincial center is further divided into sub-soums. UB City has 9 districts and 134 sub-districts.
Sample size estimation
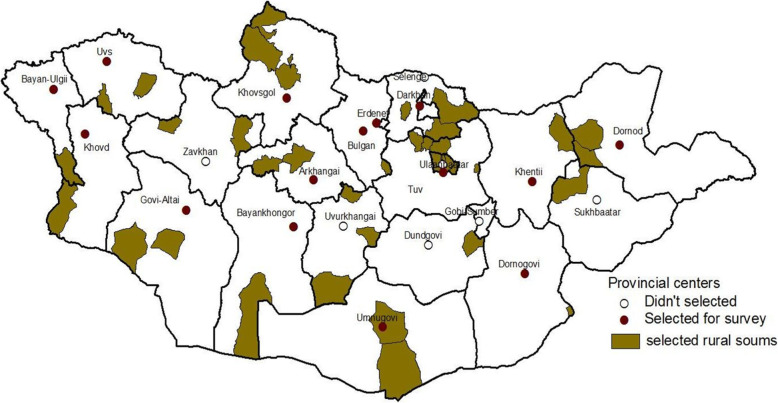
The MNTP Survey was a nationally representative population-based cross-sectional survey of households in selected clusters that aimed to investigate the prevalence of TB in Mongolia, and was conducted from April 2014 to November 2015. The 2010 Report of the Population and Housing Census conducted by the Mongolian government was used to define the sampling frame of the MNTP survey [8]. The sample size of the MNTP Survey was calculated using the WHO TB prevalence survey guidelines [23]. The survey population was divided into 3 strata according to settlement type (rural soums, provincial centers, and cities) in order to estimate the prevalence of TB. Primary sampling units (PSUs) in each stratum were defined as a soum in a rural area, a sub-soum in a provincial center, and sub-districts in UB City (Fig. (Fig.1).1). In the first stage, 98 PSUs were recruited from a list of units across Mongolia (36 from rural soums, 15 from provincial centers, and 51 from cities) using the multi-stage, random cluster sampling method. Each PSU consists of several small blocks called “clusters” consisting of ≥ 500 people aged ≥
500 people aged ≥ 15 years. Proportional probability to size sampling was used for primary sampling units, and random sampling was used for cluster sampling from a list of clusters. The required sample size for the survey was 54,100 adults from 98 clusters.
15 years. Proportional probability to size sampling was used for primary sampling units, and random sampling was used for cluster sampling from a list of clusters. The required sample size for the survey was 54,100 adults from 98 clusters.
Analyzed population
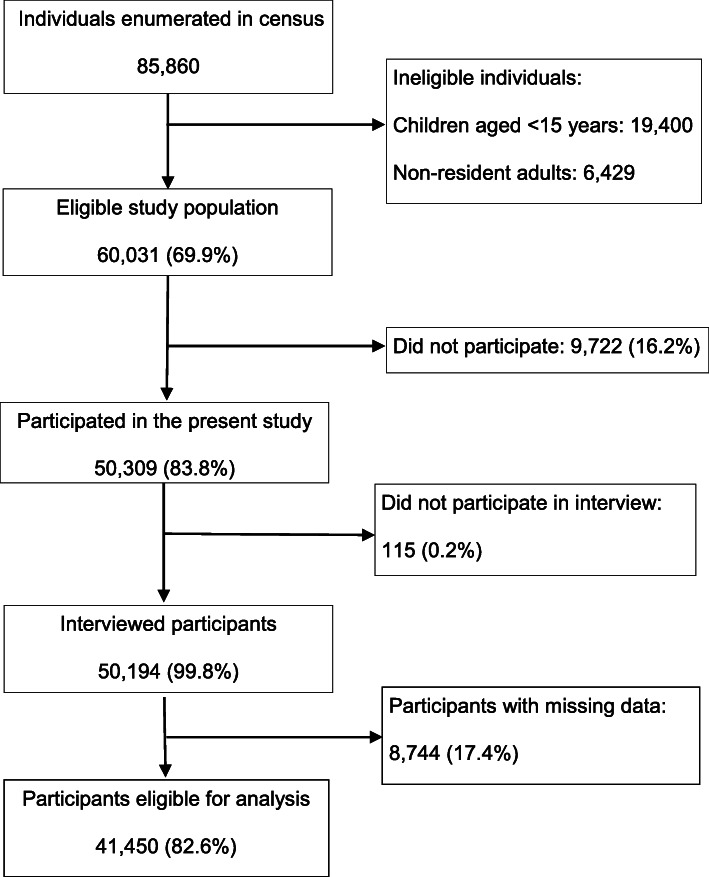
After random cluster sampling, a total of 85,860 individuals of all ages in 98 clusters were enumerated (Fig. (Fig.2).2). Among the enumerated individuals, children aged < 15 years (n = 19,400) and individuals who did not meet the residential duration criteria (n = 6,429) were excluded from the MNTP survey. Of the 60,031 eligible individuals, 50,309 (83.8%) participated in the survey and 50,194 (99.8%) were interviewed. Of the interviewed participants, 8744 with missing data on potential confounders were excluded in present study. The final study population for the present study consisted of 41,450 participants (69.0% of eligible individuals).
15 years (n = 19,400) and individuals who did not meet the residential duration criteria (n = 6,429) were excluded from the MNTP survey. Of the 60,031 eligible individuals, 50,309 (83.8%) participated in the survey and 50,194 (99.8%) were interviewed. Of the interviewed participants, 8744 with missing data on potential confounders were excluded in present study. The final study population for the present study consisted of 41,450 participants (69.0% of eligible individuals).
Questionnaire
All households in the selected clusters were visited by a survey census team. During the visit, the census team observed the indoor environment of the houses and interviewed residents about environmental factors, such as the type of housing (gher, wooden house, apartment, or other), type of heating (central heating system, furnace with solid fuels, electricity, or stove with solid fuels), type of fuel used for cooking, size of gher, and average monthly household income.
All participants were also invited to a data collection site (survey venue), where they were interviewed using a structured questionnaire, which solicited information regarding demographic and socioeconomic characteristics, TB-related symptoms, number of household members, indoor smoking, previous TB history, history of contact with an active TB case, and unhealthy habits (e.g., smoking and alcohol intake). Trained health care workers measured blood pressure, height, and body weight, with participants in light clothes without shoes. Body mass index (BMI) was calculated by dividing weight by height squared (kg/m2). All participants underwent a chest X-ray examination using direct digital radiography. All chest X-ray images taken at the collection site were interpreted by a single experienced radiologist.
TB diagnosis
Analysis of TB was based on survey case definitions according to the national TB guidelines and WHO recommendations. TB categories included smear positive TB and bacteriologically confirmed TB. Bacteriologically confirmed TB includes smear positive, smear negative but culture positive TB, and TB confirmed by a rapid diagnostic method such as the GeneXpert MTB/RIF assay. Presumptive TB was defined as a participant who had a cough for 2 weeks or longer at the time of the interview and/or any abnormality in the lung field or mediastinum detected by chest X-ray. These participants were asked to submit two sputum samples (one on the spot and one early the next morning) for laboratory confirmation by smear microscopy, the GeneXpert MTB/RIF assay, and liquid and solid cultures.
Statistical analysis
All data were anonymized and entered into an electronic database for cleaning and analysis. Bacteriologically confirmed TB, non-TB, and smear positive TB groups, and solid fuel and clean fuel users, were compared using the chi-square test for categorical variables or the unpaired t-test for continuous variables. Logistic regression models were used to identify risk factors associated with TB. To incorporate nominal independent variables into the regression model, they were transformed into dichotomous variables as follows: type of fuel used for heating was grouped into solid fuels (heating with stove or furnace) and clean fuels (municipal or electric system); marital status into single (divorced or widowed) and other (married or never married); education level into lower education (none, primary, or incomplete secondary) and other (completed secondary, technical, or higher); employment into employer, self-business owner, and other (salaried employee, member of cooperative, or unpaid participant in household enterprise); smoking status into daily smoker and other (none, quit, or occasional); alcohol consumption into yes (2–4 times a month, 2–3 times a week, or at least 4 times a week) and no (none or once a month or less); and BMI into underweight (≤ 18.5 kg/m2) and other (>
18.5 kg/m2) and other (> 18.5 kg/m2). The following variables were considered potential confounders: age, gender, education level, marital status, employment, smoking, alcohol consumption, contact with an active TB case, previous history of TB, and BMI. Given the lack of interaction effects between solid fuel use and smoking, logistic regression analysis adjusting for potential confounders was used to measure the effects of smoking, solid fuel use, and both smoking and solid fuel use on TB. Odds ratios (ORs), 95% confidence intervals (CIs), and p values were calculated. Statistical significance was defined as p<0.05 for all tests. All statistical analyses were conducted with SPSS Statistics Desktop for Japan, Version 26 (IBM Japan, Ltd., Tokyo, Japan).
18.5 kg/m2). The following variables were considered potential confounders: age, gender, education level, marital status, employment, smoking, alcohol consumption, contact with an active TB case, previous history of TB, and BMI. Given the lack of interaction effects between solid fuel use and smoking, logistic regression analysis adjusting for potential confounders was used to measure the effects of smoking, solid fuel use, and both smoking and solid fuel use on TB. Odds ratios (ORs), 95% confidence intervals (CIs), and p values were calculated. Statistical significance was defined as p<0.05 for all tests. All statistical analyses were conducted with SPSS Statistics Desktop for Japan, Version 26 (IBM Japan, Ltd., Tokyo, Japan).
Results
A total of 248 TB cases were identified in the MNTP survey. Of these, 213 TB cases, including 75 smear positive TB cases, were analyzed in our final study sample. Mean number of years living at the same residential address was 14.8 years. General characteristics by group (bacteriologically confirmed TB, smear positive TB, and non-TB) are shown in Table Table1.1. Significant differences were observed in gender, marital status, and education levels between bacteriologically confirmed TB and non-TB groups. Gender and education level significantly differed between non-TB and smear positive TB groups.
Table 1
General characteristics among study population
| Total | Non-TB | Bact TB | P valuea | Smear + TB | P valuea | |
|---|---|---|---|---|---|---|
| Gender; n (%) | ||||||
| Male | 16,902 (40.8) | 16,760 (40.6) | 142 (66.7) | < 0.01 0.01 | 61 (81.3) | < 0.01 0.01 |
| Female | 24,548 (59.2) | 24,477 (59.4) | 71 (33.3) | 14 (18.7) | ||
| Age group; n (%) | ||||||
 15–24 15–24 | 7236 (17.5) | 7202 (17.5) | 34 (16.0) | 0.50 | 8 (10.7) | 0.53 |
 25–34 25–34 | 9413 (22.7) | 9362 (22.7) | 51 (23.9) | 23 (30.7) | ||
 35–44 35–44 | 8538 (20.6) | 8501 (20.6) | 37 (17.4) | 15 (20.0) | ||
 45–54 45–54 | 8018 (19.3) | 7978 (19.3) | 40 (18.8) | 14 (18.7) | ||
 55–64 55–64 | 4998 (12.1) | 4970 (12.1) | 28 (13.1) | 9 (12.0) | ||
 65+ 65+ | 3247 (7.8) | 3224 (7.8) | 23 (10.8) | 6 (8.0) | ||
| Marital status; n (%) | ||||||
 Married Married | 29,456 (71.1) | 29,324 (71.1) | 132 (62.0) | < 0.01 0.01 | 49 (65.3) | 0.32 |
 Never married Never married | 8593 (20.7) | 8545 (20.7) | 48 (22.5) | 16 (21.3) | ||
 Divorced Divorced | 738 (1.8) | 726 (1.8) | 12 (5.6) | 3 (4.0) | ||
 Widowed Widowed | 2663 (6.4) | 2642 (6.4) | 21 (9.9) | 7 (9.3) | ||
| Education; n (%) | ||||||
 None None | 882 (2.1) | 875 (2.1) | 7 (3.3) | < 0.01 0.01 | 2 (2.7) | 0.01 |
 Primary Primary | 2242 (5.4) | 2226 (5.4) | 16 (7.5) | 7 (9.3) | ||
 Incompleted secondary Incompleted secondary | 7289 (17.6) | 7241 (17.6) | 48 (22.5) | 15 (20.0) | ||
 Completed secondary Completed secondary | 16,401 (39.6) | 16,305 (39.5) | 96 (45.1) | 40 (53.3) | ||
 Technical training Technical training | 3636 (8.8) | 3618 (8.8) | 18 (8.5) | 4 (5.3) | ||
 Higher Higher | 11,000 (26.5) | 10,972 (26.6) | 28 (13.1) | 7 (9.3) | ||
| Employed; n (%) | ||||||
 Unemployed Unemployed | 21,168 (51.1) | 21,049 (51.0) | 119 (55.9) | 0.20 | 42 (56.0) | 0.39 |
 Employed Employed | 20,282 (48.9) | 20,188 (49.0) | 94 (44.1) | 33 (44.0) | ||
| Reason unemployed; n (%) | ||||||
 Secondary school student Secondary school student | 1790 (8.5) | 1785 (8.5) | 5 (4.2) | 0.05 | 1 (2.4) | 0.09 |
 University/college student University/college student | 3133 (14.9) | 3120 (14.9) | 13 (10.9) | 3 (7.1) | ||
 Retired Retired | 6864 (32.6) | 6826 (32.6) | 38 (31.9) | 13 (31.0) | ||
 Disabled Disabled | 1634 (7.8) | 1620 (7.7) | 14 (11.8) | 7 (16.7) | ||
 Housewife Housewife | 2996 (14.2) | 2982 (14.2) | 14 (11.8) | 5 (11.9) | ||
 Cannot find job Cannot find job | 2764 (13.1) | 2741 (13.1) | 23 (19.3) | 9 (21.4) | ||
 Other Other | 1873 (8.9) | 1861 (8.9) | 12 (10.1) | 4 (9.5) | ||
| Employment; n (%) | ||||||
 Salaried employee Salaried employee | 11,972 (59.1) | 11,932 (59.2) | 40 (42.6) | 0.01 | 12 (36.4) | 0.13 |
 Employer Employer | 923 (4.6) | 914 (4.5) | 9 (9.6) | 2 (6.1) | ||
 Private business owner Private business owner | 6196 (30.6) | 6156 (30.5) | 40 (42.6) | 18 (54.5) | ||
 Member of cooperative Member of cooperative | 117 (0.6) | 117 (0.6) | 0 (0.0) | 0 (0.0) | ||
 Unpaid participant in household enterprise Unpaid participant in household enterprise | 382 (1.9) | 381 (1.9) | 1 (1.1) | 0 (0.0) | ||
 Other Other | 668 (3.3) | 664 (3.3) | 4 (4.3) | 1 (3.0) | ||
| Residence area; n (%) | ||||||
 Urban Urban | 23,283 (56.2) | 23,159 (56.2) | 124 (58.2) | 0.50 | 41 (54.7) | 0.79 |
 Rural Rural | 18,167 (43.8) | 18,078 (43.8) | 89 (41.8) | 34 (45.3) | ||
| Number of years living at same address; mean (SD) | ||||||
| 14.8 (14.1) | 14.8 (14.1) | 14.9 (13.0) | 0.30 | 15.2 (13.9) | 0.80 | |
TB tuberculosis, Bact bacteriologically confirmed, + positive, SD standard deviation
Bact TB includes smear-positive TB and culture-positive or TB approved by rapid diagnostic such as Gen Xpert/RIF
Values represent mean (SD) or N (%)
aP values were calculated using the chi-square test for categorical variables or the unpaired t test for continuous variables in order to compare differences between non-TB and Bact TB groups and between non-TB and smear + TB groups
Household environmental characteristics in bacteriologically confirmed TB, non-TB, and smear positive TB groups are shown in Table Table2.2. A majority of participants lived in a gher (32.6%), or a simple house made using wood or bricks (39.4%). The gher and simple house are not connected to a centralized heating infrastructure. Both use a simple stove or furnace to burn wood or coal for heating in the winter. With the exception of exposure to solid fuels for heating purposes, there were no significant differences in environmental factors between TB and non-TB groups, including housing type, passive tobacco smoking, presence of a separate kitchen, and average monthly household income. The distribution of households with a separate kitchen significantly differed between non-TB and smear positive TB groups. The prevalence of smear positive and bacteriologically confirmed TB cases was significantly higher in households with indoor exposure to solid fuels for heating compared to households using clean energy. Participants who smoke tobacco, drink alcohol more than twice per month, had contact with an active TB case, are underweight, and were previously diagnosed with TB were significantly more likely to have TB.
Table 2
Household environmental and individual factors among study population
| Total | Non-TB | Bact TB | P value | Smear + TB | P value | |
|---|---|---|---|---|---|---|
| House type; n (%) | ||||||
 Gher Gher | 13,531 (32.6) | 13,454 (32.6) | 77 (36.2) | 0.35 | 29 (38.7) | 0.14 |
 Wooden house Wooden house | 16,331 (39.4) | 16,243 (39.4) | 88 (41.3) | 34 (45.3) | ||
 Apartment Apartment | 10,298 (24.9) | 10,255 (24.9) | 43 (20.2) | 11 (14.7) | ||
 Other Other | 1290 (3.1) | 1285 (3.1) | 5 (2.3) | 1 (1.3) | ||
| Separate kitchen; n (%) | ||||||
 Yes Yes | 21,411 (51.7) | 21,313 (51.7) | 98 (46.0) | 0.90 | 30 (40.0) | 0.04 |
 No No | 20,039 (48.3) | 19,924 (48.3) | 115 (54.0) | 45 (60.0) | ||
| Exposure to solid fuel for heating; n (%) | ||||||
 Clean Clean | 12,069 (29.1) | 12,023 (29.2) | 46 (21.6) | 0.02 | 12 (16.0) | 0.01 |
 Solid fuel Solid fuel | 29,381 (70.9) | 29,214 (70.8) | 167 (78.4) | 63 (84.0) | ||
| Number of family members; n (%) | ||||||
 ≤ ≤ 4 4 | 25,924 (62.5) | 25,784 (62.5) | 140 (65.7) | 0.30 | 47 (62.7) | 0.98 |
 > > 5 5 | 15,526 (37.5) | 15,453 (37.5) | 73 (34.3) | 28 (37.3) | ||
| Exposure to tobacco smoke inside home, n | ||||||
 Never Never | 17,264 (41.7) | 17,181 (41.7) | 83 (39.0) | 0.70 | 28 (37.3) | 0.42 |
 Occasional Occasional | 10,554 (25.5) | 10,499 (25.5) | 55 (25.8) | 17 (22.7) | ||
 Daily Daily | 13,632 (32.9) | 13,557 (32.9) | 75 (35.2) | 30 (40.0) | ||
| Household monthly income (₮)a; n (%) | ||||||
 ≤ ≤ 500,000 500,000 | 22,155 (53.4) | 22,038 (53.4) | 117 (54.9) | 0.30 | 46 (61.3) | 0.38 |
 500,001–1,000,000 500,001–1,000,000 | 14,948 (36.1) | 14,871 (36.1) | 77 (36.2) | 25 (33.3) | ||
 1,000,001–1,500,000 1,000,001–1,500,000 | 2719 (6.6) | 2711 (6.6) | 8 (3.8) | 2 (2.7) | ||
 ≥ ≥ 1,500,001 1,500,001 | 1628 (3.9) | 1617 (3.9) | 11 (5.2) | 2 (2.7) | ||
| Smoking; n (%) | ||||||
 Never Never | 29,781 (71.9) | 29,683 (72.0) | 98 (46.0) | < 0.01 0.01 | 20 (26.7) | < 0.01 0.01 |
 Quit Quit | 1259 (3.0) | 1248 (3.0) | 11 (5.2) | 7 (9.3) | ||
 Occasional Occasional | 1396 (3.4) | 1387 (3.4) | 9 (4.2) | 5 (6.7) | ||
 Daily Daily | 9014 (21.7) | 8919 (21.6) | 95 (44.6) | 43 (57.3) | ||
| Alcohol consumption; n (%) | ||||||
 Never Never | 22,351 (53.9) | 22,266 (54.0) | 85 (39.9) | < 0.01 0.01 | 27 (36.0) | < 0.01 0.01 |
 Once a month or less Once a month or less | 15,892 (38.3) | 15,801 (38.3) | 91 (42.7) | 30 (40.0) | ||
 2-4 times a month 2-4 times a month | 2837 (6.8) | 2812 (6.8) | 25 (11.7) | 12 (16.0) | ||
 2–3 times a week 2–3 times a week | 265 (0.6) | 257 (0.6) | 8 (3.8) | 4 (5.3) | ||
 At least 4 times a week At least 4 times a week | 105 (0.3) | 101 (0.2) | 4 (1.9) | 2 (2.7) | ||
| Diabetes; n (%) | ||||||
 No No | 17,801 (43.0) | 17,717 (43.0) | 84 (39.4) | 0.09 | 32 (42.7) | 0.67 |
 Yes Yes | 1011 (2.4) | 1002 (2.4) | 9 (4.2) | 3 (4.0) | ||
 Unknown Unknown | 22,638 (54.6) | 22,518 (54.6) | 120 (56.3) | 40 (53.3) | ||
| Contact with active TB; n (%) | ||||||
 No No | 35,020 (84.5) | 34,862 (84.5) | 158 (74.2) | < 0.01 0.01 | 54 (72.0) | 0.01 |
 Yes Yes | 6430 (15.5) | 6375 (15.5) | 55 (25.8) | 21 (28.0) | ||
| BMI; n (%) | ||||||
 Normal Normal | 18,779 (45.3) | 18,627 (45.2) | 152 (71.4) | < 0.01 0.01 | 54 (72.0) | < 0.01 0.01 |
 Overweight Overweight | 13,206 (31.9) | 13,176 (32.0) | 30 (14.1) | 7 (9.3) | ||
 Obese class I Obese class I | 6096 (14.7) | 6089 (14.8) | 7 (3.3) | 1 (1.3) | ||
 Obese class II Obese class II | 1543 (3.7) | 1543 (3.7) | 0 (0.0) | 0 (0.0) | ||
 Obese class III Obese class III | 470 (1.1) | 469 (1.1) | 1 (0.5) | 0 (0.0) | ||
 Underweight Underweight | 1356 (3.3) | 1333 (3.2) | 23 (10.8) | 13 (17.3) | ||
| Previous history of TB; n (%) | ||||||
 No No | 39,785 (96.0) | 39,612 (96.1) | 173 (81.2) | < 0.01 0.01 | 52 (69.3) | < 0.01 0.01 |
 Yes Yes | 1665 (4.0) | 1625 (3.9) | 40 (18.8) | 23 (30.7) | ||
TB tuberculosis, Bact bacteriologically confirmed, ₮ tugrik
Bact TB includes smear-positive TB and culture-positive or TB approved by rapid diagnostic method such as Gen Xpert/RIF
P values were calculated using the chi-square test to compare differences between non-TB and Bact TB groups and between non-TB and smear+ TB groups
aAverage monthly household income based on tugrik (₮) Mongolian currency $1 = 1800 ₮ in 2015
Table Table33 compares the general characteristics of participants who use solid fuels and clean fuels. Participants who were aged > 25 years, who were married, who had a low level of education, who were unemployed, and who were rural residents were more likely to use solid fuels for heating than clean fuels. No significant gender difference was observed between solid fuel and clean fuel users. Household environment and individual factors significantly differed between solid fuel and clean fuel users (Table (Table4).4). Families with a lower income were more likely to use solid fuels, and families that used solid fuels were more exposed to tobacco smoke inside the home than families that used clean fuels.
25 years, who were married, who had a low level of education, who were unemployed, and who were rural residents were more likely to use solid fuels for heating than clean fuels. No significant gender difference was observed between solid fuel and clean fuel users. Household environment and individual factors significantly differed between solid fuel and clean fuel users (Table (Table4).4). Families with a lower income were more likely to use solid fuels, and families that used solid fuels were more exposed to tobacco smoke inside the home than families that used clean fuels.
Table 3
General characteristics of clean fuel users and solid fuel user
| Total | Clean fuel user | Solid fuel user | P value | |
|---|---|---|---|---|
| Gender; n (%) | ||||
 Female Female | 24,818 (59.9) | 7277 (60.3) | 17,541 (59.7) | 0.30 |
 Male Male | 16632 (40.1) | 4792 (39.7) | 11,840 (40.3) | |
| Age group; n (%) | ||||
 15–24 15–24 | 7236 (17.5) | 2678 (22.2) | 4558 (15.5) | < 0.01 0.01 |
 25–34 25–34 | 9413 (22.7) | 2660 (22.0) | 6753 (23.0) | |
 35–44 35–44 | 8538 (20.6) | 2320 (19.2) | 6218 (21.2) | |
 45–54 45–54 | 8018 (19.3) | 2106 (17.4) | 5912 (20.1) | |
 55–64 55–64 | 4998 (12.1) | 1360 (11.3) | 3638 (12.4) | |
 65+ 65+ | 3247 (7.8) | 945 (7.8) | 2302 (7.8) | |
| Marital status; n (%) | ||||
 Married Married | 29,456 (71.1) | 7918 (65.6) | 21,538 (73.3) | < 0.01 0.01 |
 Never married Never married | 8593 (20.7) | 3093 (25.6) | 5500 (18.7) | |
 Divorced Divorced | 738 (1.8) | 264 (2.2) | 474 (1.6) | |
 Widowed Widowed | 2663 (6.4) | 794 (6.6) | 1869 (6.4) | |
| Education; n (%) | ||||
 None None | 882 (2.1) | 108 (0.9) | 774 (2.6) | < 0.01 0.01 |
 Primary Primary | 2242 (5.4) | 207 (1.7) | 2035 (6.9) | |
 Incomplete secondary Incomplete secondary | 7289 (17.6) | 1146 (9.5) | 6143 (20.9) | |
 Completed secondary Completed secondary | 16,401 (39.6) | 4244 (35.2) | 12,157 (41.4) | |
 Technical training Technical training | 3636 (8.8) | 1027 (8.5) | 2609 (8.9) | |
 Higher Higher | 11,000 (26.5) | 5337 (44.2) | 5663 (19.3) | |
| Employed; n (%) | ||||
 Unemployed Unemployed | 21,168 (51.1) | 6013 (49.8) | 15,155 (51.6) | < 0.01 0.01 |
 Employed Employed | 20,282 (48.9) | 6056 (50.2) | 14,226 (48.4) | |
| Reason unemployed; n (%) | ||||
 Secondary school student Secondary school student | 1790 (8.5) | 456 (7.6) | 1334 (8.9) | < 0.01 0.01 |
 University / college student University / college student | 3133 (14.9) | 1657 (27.6) | 1476 (9.8) | |
 Retired Retired | 6864 (32.6) | 1899 (31.7) | 4965 (33.0) | |
 Disabled Disabled | 1634 (7.8) | 329 (5.5) | 1305 (8.7) | |
 Housewife Housewife | 2996 (14.2) | 740 (12.3) | 2256 (15.0) | |
 Cannot find job Cannot find job | 2764 (13.1) | 344 (5.7) | 2420 (16.1) | |
 Other Other | 1873 (8.9) | 572 (9.5) | 1301 (8.6) | |
| Employment; n (%) | ||||
 Salaried employee Salaried employee | 11,972 (59.1) | 4090 (67.7) | 7882 (55.4) | < 0.01 0.01 |
 Employer Employer | 923 (4.6) | 326 (5.4) | 597 (4.2) | |
 Private business owner Private business owner | 6196 (30.6) | 1565 (25.9) | 4631 (32.6) | |
 Member of cooperative Member of cooperative | 117 (0.6) | 11 (0.2) | 106 (0.7) | |
 Unpaid participant in household enterprise Unpaid participant in household enterprise | 382 (1.9) | 15 (0.2) | 367 (2.6) | |
 Other Other | 668 (3.3) | 36 (0.6) | 632 (4.4) | |
| Residence area; n (%) | ||||
 Urban Urban | 23,283 (56.2) | 10,550 (87.4) | 12,733 (43.3) | < 0.01 0.01 |
 Rural Rural | 18,167 (43.8) | 1519 (12.6) | 16,648 (56.7) | |
Values represent N (%)
P values were calculated using the chi-square test for categorical variables in order to compare differences between clean fuel user and solid fuel user
Table 4
Household environmental and individual factors among solid fuel user and clean fuel user
| Total | Clean fuel user | Solid fuel user | P-value | |
|---|---|---|---|---|
| Houses type; n (%) | ||||
 Gher Gher | 13531 (32.6) | 300 (2.5) | 13231 (45.0) | <0.01 |
 Wooden house Wooden house | 16331 (39.4) | 786 (6.5) | 15545 (52.9) | |
 Apartment Apartment | 10298 (24.8) | 9989 (82.8) | 309 (1.1) | |
 Other Other | 1290 (3.1) | 994 (8.2) | 296 (1.0) | |
| Separate kitchen; n (%) | ||||
 Yes Yes | 21411 (51.7) | 8982 (74.4) | 12429 (42.3) | <0.01 |
 No No | 20039 (48.3) | 3087 (25.6) | 16952 (57.7) | |
| Number of family members; n (%) | ||||
 ≤ 4 ≤ 4 | 25924 (62.5) | 7797 (64.6) | 18127 (61.7) | <0.01 |
 > 5 > 5 | 15526 (37.5) | 4272 (35.4) | 11254 (38.3) | |
| Exposure to tobacco smoke inside home; n (%) | ||||
 Never Never | 17264 (41.7) | 5231 (43.3) | 12033 (41.0) | <0.01 |
 Occasional Occasional | 10554 (25.5) | 3499 (29.0) | 7055 (24.0) | |
 Daily Daily | 13632 (32.9) | 3339 (27.7) | 10293 (35.0) | |
| Household monthly income (₮); n (%) | ||||
 ≤ 500,000 ≤ 500,000 | 22155 (53.4) | 3828 (31.7) | 18327 (62.4) | <0.01 |
 500,001-1,000,000 500,001-1,000,000 | 14948 (36.1) | 5550 (46.0) | 9398 (32.0) | |
 1,000,001-1,500,000 1,000,001-1,500,000 | 2719 (6.6) | 1576 (13.1) | 1143 (3.9) | |
 ≥ 1,500,001 ≥ 1,500,001 | 1628 (3.9) | 1115 (9.2) | 513 (1.7) | |
| Smoking; n (%) | ||||
 Never Never | 29781 (71.8) | 8756 (72.5) | 21025 (71.6) | <0.01 |
 Quit Quit | 1259 (3.0) | 380 (3.1) | 879 (3.0) | |
 Occasional Occasional | 1396 (3.4) | 504 (4.2) | 892 (3.0) | |
 Daily Daily | 9014 (21.7) | 2429 (20.1) | 6585 (22.4) | |
| Alcohol consumption; n (%) | ||||
 Never Never | 22351 (53.9) | 5860 (48.6) | 16491 (56.1) | <0.01 |
 Once a month or less Once a month or less | 15892 (38.3) | 5182 (42.9) | 10710 (36.5) | |
 2-4 times a month 2-4 times a month | 2837 (6.8) | 933 (7.7) | 1904 (6.5) | |
 2-3 times a week 2-3 times a week | 265 (0.6) | 69 (0.6) | 196 (0.7) | |
 At least 4 times a week At least 4 times a week | 105 (0.3) | 25 (0.2) | 80 (0.3) | |
| Diabetes; n (%) | ||||
 No No | 17801 (42.9) | 1484 (12.3) | 16317 (55.5) | <0.01 |
 Yes Yes | 1011 (2.4) | 387 (3.2) | 624 (2.1) | |
 Unknown Unknown | 22638 (54.6) | 10198 (84.5) | 12440 (42.3) | |
| Contact with active TB; n (%) | ||||
 No No | 35020 (84.5) | 9934 (82.3) | 25086 (85.4) | <0.01 |
 Yes Yes | 6430 (15.5) | 2135 (17.7) | 4295 (14.6) | |
| BMI; n (%) | ||||
 Normal Normal | 18779 (45.3) | 5286 (43.8) | 13493 (45.9) | <0.01 |
 Overweight Overweight | 13206 (31.9) | 3879 (32.1) | 9327 (31.7) | |
 Obese class I Obese class I | 6096 (14.7) | 1857 (15.4) | 4239 (14.4) | |
 Obese class II Obese class II | 1543 (3.7) | 484 (4.0) | 1059 (3.6) | |
 Obese class III Obese class III | 470 (1.1) | 127 (1.1) | 343 (1.2) | |
 Underweight Underweight | 1356 (3.3) | 436 (3.6) | 920 (3.1) | |
| Previous history of TB; n (%) | ||||
 No No | 39785 (96.0) | 11558 (95.8) | 28227 (96.1) | 0.10 |
 Yes Yes | 1665 (4.0) | 511 (4.2) | 1154 (3.9) | |
TB tuberculosis, BMI body mass index
P values were calculated using chi-square test to compare differences between clean fuel user and solid fuel user
aAverage monthly household income based on tugrik (₮) Mongolian currency $1 = 1800 ₮ in 2015
Table Table55 shows factors associated with bacteriologically confirmed TB and smear positive TB. Male gender, having a lower education level than secondary education, being divorced or widowed, being an employer or a private business owner, being a daily smoker, drinking alcohol more than twice a month, having contact with an active TB case, being underweight, being exposed to solid fuels for heating, and having a history of TB were significantly related to TB by univariate logistic regression analysis. In the multivariate logistic regression analysis, which included these factors plus age in the same model, exposure to solid fuels for heating was significantly associated with bacteriologically confirmed TB (OR: 1.5; 95% CI: 1.1–2.1; p = 0.02) and smear positive TB (OR = 2.1; 95% CO: 1.1–4.0; p = 0.01). Figure Figure33 shows the adjusted ORs (aORs) of smoking, exposure to solid fuels for heating, or exposure to both for bacteriologically confirmed TB. Both exposure to smoke from tobacco and solid fuels for heating were significantly associated with bacteriologically confirmed TB after adjusting for age, gender, marital status, education level, employment, being underweight, alcohol consumption, contact with an active TB case, and previous history of TB.
Table 5
The factors associated with bacteriologically confirmed TB and smear positive TB among study population
| Independent variables | Prevalence of Bact TB (%) | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|---|
| Bact TB | Bact TB | Smear + TB | |||||
| cOR (95%, CI) | P value | aOR (95%, CI) | P valuea | aOR (95%, CI) | P valuea | ||
| Gender | |||||||
 Male Male | 0.8 | 2.9 (2.2–3.9) | 0.01 | 2.2 (1.6–3.1) | < 0.01 | 4.2 (2.3–8.2) | < 0.01 |
 Female Female | 0.3 | 1.0 | – | 1.0 | – | 1.0 | – |
| Exposure to solid fuel for heating | |||||||
 Yes Yes | 0.6 | 1.5 (1.1–2.1) | 0.01 | 1.5 (1.1–2.1) | 0.02 | 2.1 (1.1–4.0) | 0.01 |
 No No | 0.4 | 1.0 | – | 1.0 | – | 1.0 | – |
| Education | |||||||
 Lower than incomplete secondary Lower than incomplete secondary | 0.7 | 1.5 (1.1–2.0) | 0.01 | 1.1 (0.9–1.6) | 0.35 | 1.0 (0.6–1.7) | 0.98 |
 Higher than complete secondary Higher than complete secondary | 0.5 | 1.0 | – | 1.0 | – | 1.0 | – |
| Marital status | |||||||
 Divorced or widow Divorced or widow | 1.0 | 2.1 (1.4–3.0) | 0.01 | 2.5 (1.6–3.8) | 0.01 | 2.7 (1.3–5.6) | 0.01 |
 Married or never married Married or never married | 0.5 | 1.0 | – | 1.0 | – | 1.0 | – |
| Employment | |||||||
 Employer and self-business owner Employer and self-business owner | 0.7 | 1.4 (1.1–2.0) | 0.03 | 1.4 (0.9–1.9) | 0.06 | 1.4 (0.8–2.4) | 0.23 |
 Others Others | 0.5 | 1.0 | – | 1.0 | – | 1.0 | – |
| Smoking | |||||||
 Daily Daily | 1.1 | 2.9 (2.2–3.8) | 0.01 | 1.8 (1.3–2.5) | < 0.01 | 2.1 (1.2–3.5) | 0.01 |
 Never, quit, occasional Never, quit, occasional | 0.4 | 1.0 | – | 1.0 | – | 1.0 | – |
| Alcohol intake | |||||||
 Yes Yes | 1.2 | 2.5 (1.8–3.6) | < 0.01 | 1.4 (0.9–2.1) | 0.07 | 1.5 (0.9–2.8) | 0.13 |
 No No | 0.5 | 1.0 | – | 1.0 | – | 1.0 | – |
| Contacts with active TB | |||||||
 Yes Yes | 0.9 | 1.9 (1.4–2.6) | < 0.01 | 1.7 (1.2–2.3) | 0.01 | 1.6 (0.9–2.7) | 0.13 |
 No No | 0.5 | 1.0 | – | 1.0 | – | 1.0 | – |
| Underweight | |||||||
 BMI ≤ 18.5 kg/m2 BMI ≤ 18.5 kg/m2 | 1.7 | 3.6 (2.3–5.6) | < 0.01 | 3.6 (2.3–5.7) | < 0.01 | 7.1 (3.7–13.5) | < 0.01 |
 BMI > 18.5 kg/m2 BMI > 18.5 kg/m2 | 0.5 | 1.0 | – | 1.0 | – | 1.0 | – |
| History of TB | |||||||
 Yes Yes | 2.4 | 5.6 (4.0–8.0) | < 0.01 | 4.3 (3.0–6.2) | < 0.01 | 7.5 (4.4–12.6) | < 0.01 |
 No No | 0.4 | 1.0 | – | 1.0 | – | 1.0 | – |
TB tuberculosis, Bact bacteriologically confirmed, + positive, cOR crude odds ratio, aOR adjusted odds ratio, CI confidence interval, BMI body mass index
Smoking: yes: tobacco smoking daily; alcohol consumption: yes: more than 2–4 times per month
BMI: underweight: ≤ 18.5 kg/m2. Adjusted effects of the independent variables on TB in the study population
Both crude and adjusted odds ratio were estimated by logistic regression. Unadjusted odds ratios were based on separate logistic regression for independent variables
TB case, underweight status, previous history of tuberculosis and exposure to solid fuel for heating
aAll independent variables were included in the same model: age, gender, education level, marital status, employment, smoking, alcohol intake, contact with active TB case, underweight status, previous history of tuberculosis and exposure to solid fuel for heating
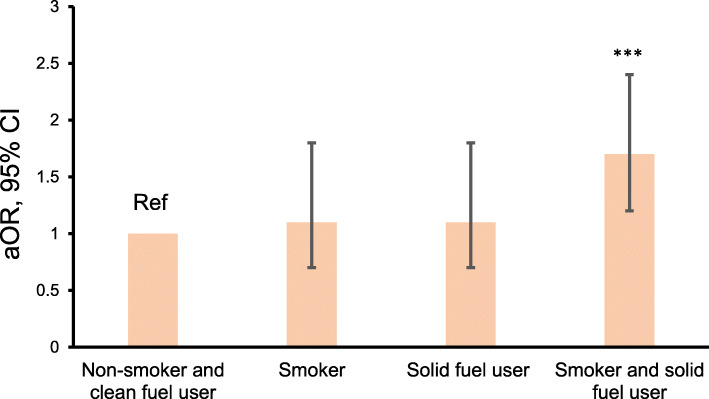
Combined effect of smoking and exposure to solid fuels on TB. Adjusted odds ratios were estimated by logistic regression after adjusting for age, gender, education level, marital status, employment, alcohol consumption, contact with an active TB case, underweight status, and previous history of tuberculosis. P < 0.05 was considered statistically significant. ***P < 0.01, compared with non-smoking clean fuel user. aOR adjusted odds ratio, CI confidence interval, % percentage
Discussion
The present large-scale study of a representative Mongolian adult population found a significant positive association between exposure to solid fuels for heating and TB. This association was independent of potential confounding factors, such as gender, smoking, marital status, BMI, contact with an active TB case, and previous history of TB. IAP from household solid fuel combustion may be a risk factor for TB in the Mongolian population, which spends most of the time at home indoors due to the cold climate.
In 2017, household IAP contributed to 1.8 million global deaths and 60.9 million disability adjusted life years (DALYs), and infectious respiratory diseases including TB accounted for most of the respiratory burden, with 27.4 million DALYs [7]. Although TB and IAP are both pressing public health issues in Mongolia, the present study is the first to report an association between IAP due to solid fuel combustion and TB in Mongolia. Due to extreme cold and long heating season, it is common for households to keep their doors and windows closed, which reduces the air circulation indoors, concentration of the pollutants released from burning solid fuels increases the exposure to respirable pollutants on individual level. Prolonged exposure to such pollutants impairs the normal clearance of secretions on the tracheobronchial mucosal surface and thus may allow a causative organism mycobacterium TB, to escape the first level of host defenses which prevent bacilli from reaching the alveoli [5].
Given that people spend 90% of their lifetime in indoor settings, indoor air quality is a major risk factor for human health [24, 25]. Some epidemiological studies have reported an association between solid fuel smoke and TB. In a case-control study conducted in Mexico, household IAP exposure was found to facilitate the development of active TB, and exposure to smoke from biomass fuels for more than 20 years led to a 3-fold higher incidence of active TB than controls (OR: 3.3; 95% CI: 1.06–10.30) [26]. A hospital-based case-control study by Pokhrel et al. found that exposure to IAP was 3.4 times more common in TB cases than in controls [27]. A meta-analysis, which included a systematic review of 12 papers, reported a 30% higher risk of developing TB in individuals exposed to IAP (OR: 1.30; 95% CI: 1.04–1.62; p < 0.02) [19]. Another meta-analysis concluded that the risk of active TB depends on the type of fuel used, with the highest risk (43% increased risk) being associated with burning solid fuels [25]. A recent meta-analysis reported that IAP is associated with the risk of contracting TB (relative risk: 1.68; 95%, CI: 1.108–2.542; p < 0.014) [28].
The combustion of solid fuels emits many chemicals which impact human health, including PM, carbon dioxide, carbon monoxide, sulfur dioxide (SO2), sulfur trioxide, nitrogen dioxide, and nitric oxide [29]. There is increasing evidence that PM exposure weakens anti-mycobacterial host immunity [30, 31]. Chronic PM exposure accompanied by high constitutive expression of pro-inflammatory cytokines results in relative cellular unresponsiveness [31, 32]. Eighty percent of the total global exposure to airborne PM occurs indoors in developing countries [33]. PM2.5 has been reported to affect lung pathology, with smear positive TB patients being more exposed to PM2.5 than smear negative TB patients [34]. Moreover, chronic exposure to PM10 ≥ 50 μg/m3 was associated with an increase in the time required for TB positive sputum culture conversion [35]. In the present study, people exposed to IAP from household solid fuel use were more likely to have smear positive TB than bacteriologically confirmed TB, and exposure to smoke from tobacco were also associated with bacteriologically confirmed TB. The indoor PM2.5 concentration is very high in ghers and houses with stoves using semi-coke coal, with estimates of 107.0 μg/m3 in winter months average, which is higher than the permissible concentration in the WHO air quality guidelines (i.e., not exceed 10 μg/m3 annual mean or 25 μg/m3 24-h mean) [36]. SO2 is also a major pollutant from solid fuel combustion, and SO2 from coal burning is associated with persistent cough symptoms among schoolchildren in urban and suburban Mongolia [37].
50 μg/m3 was associated with an increase in the time required for TB positive sputum culture conversion [35]. In the present study, people exposed to IAP from household solid fuel use were more likely to have smear positive TB than bacteriologically confirmed TB, and exposure to smoke from tobacco were also associated with bacteriologically confirmed TB. The indoor PM2.5 concentration is very high in ghers and houses with stoves using semi-coke coal, with estimates of 107.0 μg/m3 in winter months average, which is higher than the permissible concentration in the WHO air quality guidelines (i.e., not exceed 10 μg/m3 annual mean or 25 μg/m3 24-h mean) [36]. SO2 is also a major pollutant from solid fuel combustion, and SO2 from coal burning is associated with persistent cough symptoms among schoolchildren in urban and suburban Mongolia [37].
In Mongolia, 45.2% of households live in traditional ghers and 29.5% live in ordinary wooden houses [8]. Over 95% of households living in ghers use solid fuels including coal for everyday cooking and heating [38]. The traditional gher is a portable circular wood framed dwelling covered in multiple layers of wool felt. Heating is provided by a stove located at the center of the gher, and a chimney directs the fuel smoke through the central roof vent. In Mongolia, TB cases show seasonality, sharply rising in the spring from March to May. UB is the coldest capital city in the world, with temperatures reaching minus 40 °C during the night in winter. People spend most of their time indoors, and thus transmissibility of TB increases, as people are exposed to solid fuel smoke at home [39, 40].
We also found that TB is more common among males than females, and that tobacco smoking is associated with TB. Compared to non-smokers, smokers have an increased risk of developing TB (OR: 1.8; 95% CI: 1.3–2.5; p < 0.01). This finding is consistent with previous studies. In addition, there was positive association between tobacco smoking and solid fuel use in the present study. Therefore, tobacco smoking may be one of potential confounding factors. According to a WHO report, the global TB incidence is higher in males than in females, with male-to-female ratios of TB ranging from 1.3 in the Eastern Mediterranean to 2.1 in the Western Pacific region [3]. This gender difference in incidence might be explained by the higher rate of smoking among Mongolian men than women (males, 46.3%; females, 6.8%) [41]. In the present study, smoking was more prevalent in males compared to females (males, 44%; females, 6%). Plenty of epidemiological and biological studies provide insight into the biological mechanism underlying the association between tobacco smoking and TB. Tobacco smoke exposure attenuates cytokine production and TB killing by macrophages, and exposure to nicotine impairs the anti-TB defense of macrophages by two mechanisms, including the inhibition of autophagy and activation of immunosuppressive Treg cells [42].
Numerous epidemiological studies demonstrate that both the exposure from active and passive smoking have been shown to be associated with TB infection and the transmission from being infected to developing TB disease [43–45]. However, our present study could not find the relationship between passive tobacco exposure and TB association. Passive smoking exposure is lower than that experienced by active smokers, while the smoke is generally similar and contains the same gases and particles including a wide range of irritating compounds and carcinogens [46]. Indoor PM2.5 concentrations can become extremely high when burning solid fuels than tobacco smoking, therefore a relatively small effect size might also partly explain why it has previously proved so difficult to establish such a relationship in this study.
The present study has several strengths. First, we used data from a nationally representative population-based survey which targeted households throughout the country. Thus, our sample size was very large, reducing potential type 2 error. Second, detailed information about potential risk factors for TB were recorded, allowing us to comprehensively adjust for confounders. Third, TB was diagnosed by laboratory test results rather than subjectively by self-report or based on a clinically-diagnosed previous history of the disease.
The present study also has some limitations worth noting. First, as with other observational studies, associations observed may be due to unmeasured confounders. However, the associations between household solid fuel use and TB reported in the present study were independent of other potential confounders such as smoking, gender, marital status, education, alcohol intake, BMI, contact with an active TB case, and previous history of TB. Second, we adopted a cross-sectional design. Data on assessed variables were obtained only at the time of recruitment, and thus the duration of risk factors and its impact to the individual’s level could not be assessed. Moreover, the exposure to solid fuel smoke from cooking and heating was self-reported, and the duration of exposure to solid fuels and concentration of pollutants in indoor settings were not measured. That said, the study team visited every household and confirmed the type of dwelling and stoves used in order to minimize recall bias.
Conclusion
This large, population-based cross-sectional analysis showed that exposure to solid fuels for heating is associated with active TB, including smear positive TB, independently of several confounding factors, in Mongolian adults. Moreover, the combination of smoking and solid fuel use for heating is associated with developing active TB. A greater awareness of and more education on the use of solid fuels is needed, given its relevance as a source of IAP and relationship with TB.
Acknowledgements
The authors thank the MNTP Survey team and staff members of the Mongolian National Center for Communicable Disease.
Abbreviations
| aOR | Adjusted odds ratio |
| BMI | Body mass index |
| DALY | Disability adjusted life years |
| HIV | Human immunodeficiency virus |
| IAP | Indoor air pollution |
| MNTP Survey | Mongolian National Tuberculosis Prevalence Survey |
| OR | Odds ratio |
| PM | Particulate matter |
| PSUs | Primary sampling units |
| SO2 | Sulfur dioxide |
| TB | Tuberculosis |
| UB | Ulaanbaatar |
| WHO | World Health Organization |
Authors’ contributions
Study conception and design: MD, KK, ND, TB, and TS. Acquisition of data: MD and TB. Analysis and interpretation: MD and KK. Drafting manuscript or revising it critically for important intellectual content: MD, KK, ND, TB, TS, CN, and TN. Writing the manuscript: MD and KK. Approving the final version of manuscript: MD, KK, ND, TB, TS, CN, and TN.
Availability of data and materials
The dataset generated during the current study is not publicly available, but is available from the corresponding author on reasonable request.
Declarations
The protocol of the present survey was approved by the Ethics Committee of the Ministry of Health of Mongolia (reference No. 04). Analysis of the secondary data set with no identifiable information on survey participants at Kansai Medical University was approved by the Ethics Committee of Kansai Medical University (reference No. 2019278). Trained healthcare workers explained the purpose of the survey to participants or their guardians (if participants were aged < 16 years), and written consent was obtained. All participants were allowed to decline participation on their own accord, even during the interview or examination.
16 years), and written consent was obtained. All participants were allowed to decline participation on their own accord, even during the interview or examination.
Not applicable.
The authors declare that they have no competing interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Articles from Environmental Health and Preventive Medicine are provided here courtesy of The Japanese Society for Hygiene
Full text links
Read article at publisher's site: https://doi.org/10.1186/s12199-021-00996-4
Read article for free, from open access legal sources, via Unpaywall:
https://environhealthprevmed.biomedcentral.com/counter/pdf/10.1186/s12199-021-00996-4
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/112510337
Article citations
Indoor Solid Fuel Use and Non-Neoplastic Digestive System Diseases: A Population-Based Cohort Study Among Chinese Middle-Aged and Older Population.
Int J Public Health, 67:1605419, 21 Dec 2022
Cited by: 1 article | PMID: 36618433 | PMCID: PMC9810631
Correction to: Figure 3 in Association between household solid fuel use and tuberculosis: cross-sectional data from the Mongolian National Tuberculosis Prevalence Survey.
Environ Health Prev Med, 27:50, 01 Jan 2022
Cited by: 0 articles | PMID: 36543228 | PMCID: PMC9792560
Correction to: Association between household solid fuel use and tuberculosis: cross-sectional data from the Mongolian National Tuberculosis Prevalence Survey.
Environ Health Prev Med, 26(1):87, 07 Sep 2021
Cited by: 1 article | PMID: 34493203 | PMCID: PMC8425153
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Effect of indoor air pollution from biomass and solid fuel combustion on prevalence of self-reported asthma among adult men and women in India: findings from a nationwide large-scale cross-sectional survey.
J Asthma, 49(4):355-365, 07 Mar 2012
Cited by: 44 articles | PMID: 22397465 | PMCID: PMC5560475
Indoor solid fuel use and tuberculosis in China: a matched case-control study.
BMC Public Health, 11:498, 25 Jun 2011
Cited by: 19 articles | PMID: 21702987 | PMCID: PMC3141461
Long-term exposure to indoor air pollution and risk of tuberculosis.
Indoor Air, 31(3):628-638, 23 Oct 2020
Cited by: 3 articles | PMID: 33016379 | PMCID: PMC9580027
Household air pollution from domestic combustion of solid fuels and health.
J Allergy Clin Immunol, 143(6):1979-1987, 01 Jun 2019
Cited by: 78 articles | PMID: 31176380
Review

 1
1