Abstract
Free full text

Comparison of Transvalvular Aortic Mean Gradients Obtained by Intraprocedural Echocardiography and Invasive Measurement in Balloon and Self‐Expanding Transcatheter Valves
Associated Data
Abstract
Background
Concerns about discordance between echocardiographic and invasive mean gradients after transcatheter aortic valve replacement (TAVR) with balloon‐expandable valves (BEVs) versus self‐expanding valves (SEVs) exist.
Methods and Results
In a multicenter study, direct‐invasive and echocardiography‐derived transvalvular mean gradients obtained before and after TAVR were compared as well as post‐TAVR and discharge echocardiographic mean gradients in BEVs versus SEVs in 808 patients. Pre‐TAVR, there was good correlation (R=0.614; P<0.0001) between direct‐invasive and echocardiography‐derived mean gradients and weak correlation (R=0.138; P<0.0001) post‐TAVR. Compared with post‐TAVR echocardiographic mean gradients, both valves exhibit lower invasive and higher discharge echocardiographic mean gradients. Despite similar invasive mean gradients, a small BEV exhibits higher post‐TAVR and discharge echocardiographic mean gradients than a large BEV, whereas small and large SEVs exhibit similar post‐TAVR and discharge mean gradients. An ejection fraction <50% (P=0.028) and higher Society of Thoracic Surgeons predicted risk of mortality score (P=0.007), but not invasive or echocardiographic mean gradient ≥10 mm Hg (P=0.378 and P=0.341, respectively), nor discharge echocardiographic mean gradient ≥20 mm Hg (P=0.393), were associated with increased 2‐year mortality.
Conclusions
Invasively measured and echocardiography‐derived transvalvular mean gradients correlate well in aortic stenosis but weakly post‐TAVR. Post‐TAVR, echocardiography overestimates transvalvular mean gradients compared with invasive measurements, and poor correlation suggests these modalities cannot be used interchangeably. Moreover, echocardiographic mean gradients are higher on discharge than post‐TAVR in all valves. Despite similar invasive mean gradients, a small BEV exhibits higher post‐TAVR and discharge echocardiographic mean gradients than a large BEV, whereas small and large SEVs exhibit similar post‐TAVR and discharge mean gradients. Immediately post‐TAVR, elevated echocardiographic‐derived mean gradients should be assessed with caution and compared with direct‐invasive mean gradients. A low ejection fraction and higher Society of Thoracic Surgeons score, but not elevated mean gradients, are associated with increased 2‐year mortality.
Nonstandard Abbreviations and Acronyms
- AS
- aortic stenosis
- BEV
- balloon‐expandable valve
- SEV
- self‐expanding valve
- TAVR
- transcatheter aortic valve replacement
Transcatheter aortic valve replacement (TAVR) with balloon‐expandable valve (BEV) and self‐expanding valve (SEV) platforms are approved in patients with severe native aortic stenosis (AS) 1 , 2 , 3 , 4 , 5 , 6 , 7 and degenerated surgical bioprosthetic valves (surgical aortic valve replacement). 8
Echocardiography has been established as the primary method for evaluation of prosthetic valve performance, and several studies have reported the post‐TAVR transvalvular mean gradients and the aortic valve area in currently approved BEVs and SEVs. 9 , 10 , 11 , 12 , 13 , 14 Discharge echocardiographic mean gradients remain a parameter for defining TAVR device success or failure 12 as well as a measure of comparative hemodynamic performance between different TAVR valves, despite an unclear impact on clinical outcomes. 15
Discordance between direct‐invasive and echocardiography‐derived mean gradients has been reported in native AS, 16 in normal surgical aortic valve replacement valves, 17 , 18 , 19 , 20 , 21 and following TAVR and valve‐in‐valve TAVR. 22 , 23 Echocardiographic mean gradients, derived from the transaortic velocity, are subject to simplification of, and inherent limitations within, the Bernoulli equation when it is applied in otherwise normal prosthetic valves combined with the omission of pressure recovery. 16 , 17 , 18 , 19 , 20 , 21 , 24 Assuming increased transaortic velocity occurs only from an increased gradient, while ignoring other causes of increased velocity, echocardiography causes significant overestimation of transvalvular mean gradients compared with invasive measures.
In this multicenter study, we sought to compare directly measured invasive mean gradients with their respective echocardiography‐derived transaortic valve mean gradients obtained intraprocedurally at baseline pre‐TAVR and immediately post‐TAVR, to compare SEVs versus BEVs in regard to post‐TAVR invasive, echocardiographic, and discharge echocardiographic mean gradients, and to assess the impact of elevated mean gradients on clinical outcomes. We hypothesized that echocardiographic‐derived mean gradients would correlate favorably to invasive mean gradients in patients with severe AS before TAVR, but not in normal functioning prosthesis post‐TAVR.
METHODS
Study Design
This is a multicenter study of consecutive patients undergoing TAVR at: Ascension St. Mary's Hospital, Saginaw, MI; Beaumont Hospital, Royal Oak, MI; Bern University Hospital, Bern, Switzerland; Delray Medical Center, Delray Beach, FL; The Heart Hospital Baylor Plano, Plano, TX; Pima Heart and Vascular, Tucson Medical Center, Tucson, AZ; Massachusetts General Hospital, Boston, MA; Quebec Heart and Lung Institute, Quebec City, Quebec, Canada; and University of Florida, Gainesville, FL.
Each participating center included patients' clinical, echocardiography, invasive, and valve data into a multicenter registry. The inclusion criteria for this analysis were patients who underwent TAVR for severe AS with available post‐TAVR echocardiography and invasive mean gradients. Beaumont Hospital was the coordinating center, and A.A. had access to all the data entries after submission by participants and was responsible for data integrity. Each institution's institutional review board approved the study, protected health information was removed from the data, and data‐sharing agreements were signed between the coordinating center and participating institutions. Authors elect not to make data available because of ongoing analysis and studies.
Clinical Data
Patients' demographics, mortality date, and time to follow‐up were retrieved from the medical records by each center. Clinical data points included body surface area, body mass index, comorbidities, New York Heart Association class at baseline, and Society of Thoracic Surgeons predicted risk of mortality.
Echocardiography
Echocardiograms were acquired using a commercially available ultrasound machine, and measurements were done according to current recommendations. 25 , 26 The left ventricular outflow tract (LVOT) diameter was measured immediately beneath the aortic valve leaflets before TAVR, and just below the left ventricular border of the bioprosthetic valve stent from outer‐to‐outer border in a parasternal long‐axis zoom view post‐TAVR and at discharge. However, if the apical margin of the stent was too low, the LVOT diameter was measured within the proximal portion of the valve stent. 14 , 27 The pulse‐wave Doppler sample volume was positioned just apical to the prosthesis stent at approximately the same location as the LVOT diameter measurement to obtain the LVOT time‐velocity integral. Stroke volume was calculated by multiplying LVOT area (calculated from LVOT diameter) by LVOT velocity‐time integral and was indexed for body surface area to obtain stroke volume index. The aortic valve area was calculated by the continuity equation and indexed by body surface area to obtain the indexed aortic valve area. The mean transvalvular gradient was derived from the modified Bernoulli formula through tracing the continuous wave Doppler jet across the valve from multiple windows. The ejection fraction was obtained by the Simpson biplane methods, as previously described, 26 and aortic regurgitation was calculated as a combination of both paravalvular and transvalvular regurgitation.
Invasive Gradient
The transvalvular mean gradient was obtained immediately before and after valve deployment as follows. The left ventricular (LV) pressure was obtained as apical as possible in the LV, either from a pigtail introduced into the LV, via the delivery system catheter in the LV after wire removal, or via the LV lumen of a dual‐lumen catheter. The aortic pressure was obtained in the ascending aorta from another pigtail catheter via contralateral access, via the delivery system catheter positioned in the ascending aorta, or via the aortic lumen of a dual‐lumen catheter.
Simultaneous LV and aortic pressures were obtained, both transducers were adequately flushed and zeroed, and waveforms were checked for dampening before recording.
Absolute and Percentage Differences Between Echocardiographic and Invasive Mean Gradients (Discordance)
The absolute difference between pre‐TAVR and post‐TAVR echocardiographic and invasive mean gradients was calculated as follows: (echocardiography−invasive mean gradient) and used as a measure of absolute discordance.
The percentage difference between pre‐TAVR and post‐TAVR echocardiographic and invasive mean gradients was calculated as follows: ([echocardiography−invasive mean gradient]/echocardiographic mean gradient)×100 and used as a measure of percentage discordance.
Absolute and Percentage Differences Between Post‐TAVR and Discharge Echocardiographic Mean Gradients
Absolute difference between echocardiographic post‐TAVR and discharge mean gradients was calculated as follows: (discharge mean gradient−post‐TAVR mean gradient).
Percentage difference between echocardiographic post‐TAVR and discharge mean gradients was calculated as follows: ([discharge mean gradient−post‐TAVR mean gradient]/post‐TAVR mean gradient)×100.
Valve Types and Sizes
Valves were classified as small based on the sizes of different valve types fitting an annular area ≤415 mm2 per the manufacturers' recommendation. For BEVs, large included 26 and 29 mm, and small included 20 and 23 mm. For SEVs, large included 29, 31, and 34 mm, and small included 23 and 26 mm.
Statistical Analysis
Continuous data were presented as mean±SD or as median and interquartile range, when distribution was skewed as for the mean gradients. Categorical data were presented as percentages and fraction of occurrence. Pre‐TAVR and post‐TAVR echocardiographic versus invasive mean gradients and post versus discharge echocardiographic mean gradients based on valve types and sizes were compared using the dependent t test for normally distributed variables and Wilcoxon signed‐rank test for nonparametric variables. Comparisons between absolute and percentage discordance between pre‐TAVR and post‐TAVR were conducted only in patients with both concomitant pre‐TAVR echocardiographic and invasive mean gradients and post‐TAVR echocardiographic and invasive mean gradients. Comparisons between post‐TAVR and discharge echocardiographic mean gradients were conducted only in patients with both post‐TAVR and discharge echocardiographic mean gradients.
Group comparisons of the above indexes between valve types and sizes were performed using the independent t test, or Mann‐Whitney U test for nonparametric data. Pearson correlation and linear regression analysis were conducted between invasive and echocardiographic mean gradients pre‐TAVR and post‐TAVR. Deming regression was also performed to account for potential measurement errors with either method; an error ratio of 1 was assumed. Passing‐Bablok regression was also performed given the nonparametric and heteroscedastic data post‐TAVR, as well as relative resistance to the effect of outliers.
Kaplan‐Meier estimates and log‐rank test were used to compare occurrence of mortality over 2 years, stratified according to echocardiographic and invasive mean gradients, ejection fraction following TAVR, and Society of Thoracic Surgeons predicted risk of mortality.
All statistical analyses were performed with the use of SPSS software, version 25.0 (IBM, New York, NY) and R version 4.0.1 in R‐studio version 1.3.959 (R Foundation for Statistical Computing, Vienna, Austria). P<0.05 was considered statistically significant. There was no adjustment for multiplicity.
Results
A total of 808 patients with AS underwent TAVR with intraprocedural invasive and echocardiographic mean gradients obtained (Figure 1); 629 (78%) were BEVs (214 [34%] small and 414 [66%] large), and 179 (22%) were SEVs (68 [38%] small and 109 [62%] large); size was not available in 3 patients. Intraprocedural pre‐TAVR mean gradients were obtained and only available in 547 of 808 patients (68%), and discharge echocardiographic mean gradients were obtained and only available in 744 of 808 patients (92%).
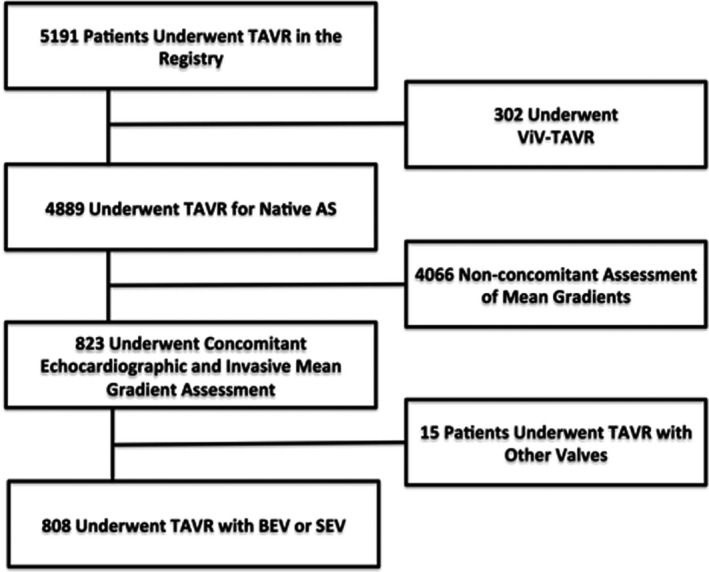
ViV indicates valve in valve.
Echocardiographic and invasive measurements were obtained within 15 to 20 minutes pre‐TAVR, within 5 to 10 minutes post‐TAVR, and before discharge for discharge echocardiography. Table 1 demonstrates baseline demographics and hemodynamics.
Table 1
Baseline Clinical, Echocardiographic, Invasive, and CTA Characteristics
| Baseline Characteristics (N=808) | Findings, Mean±SD or No. (%) |
|---|---|
| Clinical | |
| Age, y (n=782) | 81±9 |
| Male sex (n=798) | 428 (53) |
| Body mass index, kg/m2 (n=709) | 29±7 |
| Body surface area, m2 (n=748) | 1.9±0.27 |
| Hypertension (n=769) | 482 (63) |
| Diabetes mellitus (n=769) | 390 (51) |
| Dyslipidemia (n=645) | 550 (85) |
| Chronic kidney disease (n=444) | 306 (69) |
| Coronary artery disease (n=769) | 251 (33) |
| NYHA class (n=769) | 2.45±0.76 |
| I–II | 375 (49) |
| III–IV | 394(51) |
| Society of Thoracic Surgery predicted risk of mortality (n=734) | 5.82±4.3 |
| <4 | 263 (6) |
| 4–8 | 341 (6) |
| >8 | 130 (18) |
| Echocardiographic hemodynamics | |
| Ejection fraction, % (n=760) | 57±13 |
| >50% | 604 (79) |
| <50% | 156 (21) |
| Mean gradient, mm Hg (n=643) | 41±13 |
| AVA, cm2 (n=452) | 0.70±0.25 |
| Indexed AVA, cm2/m2 (n=438) | 0.37±0.13 |
| SVI, mL/m2 (n=325) | 33±11 |
| Low‐flow (SVI <35 mL/m2) | 196 (60) |
| Invasive mean gradient, mm Hg (n=627) | 36±14 |
| Cardiac CTA | |
| CT annular area, mm2 | 463±92 |
| CT annular perimeter, mm | 78±8 |
Data are reported as mean±SD for continuous variables and as number (percentage) of occurrence for categorical variables. AVA indicates aortic valve area; CT, computed tomography; CTA, CT angiography; NYHA, New York Heart Association; and SVI, stroke volume index.
Discordance Between Echocardiographic and Invasive Mean Gradients Pre‐TAVR Versus Post‐TAVR
Invasive and echocardiographic mean gradients are reported as median (interquartile range). Table 2 demonstrates post‐TAVR and discharge valve hemodynamics.
Table 2
Post‐TAVR and Discharge Invasive and Echocardiographic Hemodynamics
| Variables (N=808) |
Findings, Mean±SD or No. (%) |
|---|---|
| Post‐TAVR hemodynamics | |
| Echocardiographic mean gradient, mm Hg (n=808) | 5±3 |
| >10 mm Hg | 57 (7) |
| >20 mm Hg | 2 (0.2) |
| Invasive mean gradient, mm Hg (n=808) | 2±3 |
| >5 mm Hg | 105 (13) |
| >10 mm Hg | 21 (3) |
| AVA, cm2 (n=532) | 1.95±0.63 |
| Indexed AVA, cm2/m2 (n=521) | 1.04±0.34 |
| Severe PPM | 54 (10) |
| SVI, mL/m2 (n=322) | 31±11 |
| Low‐flow (SVI <35 mL/m2) | 222 (69) |
| Discharge TAVR hemodynamics | |
| Echocardiographic mean gradient, mm Hg (n=744) | 10±4 |
| >20 mm Hg | 30 (4) |
| AVA, cm2 (n=495) | 1.7±0.54 |
| Indexed AVA, cm2/m2 (n=491) | 0.9±0.29 |
| Severe PPM | 83 (17) |
| SVI, mL/m2 (n=621) | 37±11 |
| Low‐flow (SVI <35 mL/m2) | 280 (45) |
| Ejection fraction, % (n=621) | 60±13 |
| >50% | 522 (84) |
| <50% | 99 (16) |
| Aortic regurgitation (n=626) | |
| Aortic regurgitation >2 | 32 (5) |
| Valve type (n=808) | |
| Self‐expanding valve | 179 (22) |
| Small | 68 (38) |
| Large | 109 (62) |
| Balloon‐expanding valve | 629 (78) |
| Small | 214 (34) |
| Large | 414 (66) |
Data are reported as mean±SD for continuous variables and as number (percentage) of occurrence for categorical variables. AVA indicates aortic valve area; PPM, prosthesis patient mismatch; SVI, stroke volume index; and TAVR, transcatheter aortic valve replacement.
In patients with AS before TAVR, Deming and Passing‐Bablok regression reveal good correlation (Pearson r=0.614; P<0.0001) (Figure 2A and 2C), and significant difference between echocardiographic (41 [33–49] mm Hg) versus invasive mean gradients (35 [26–44] mm Hg; P<0.0001) was noted (Figure 2E).
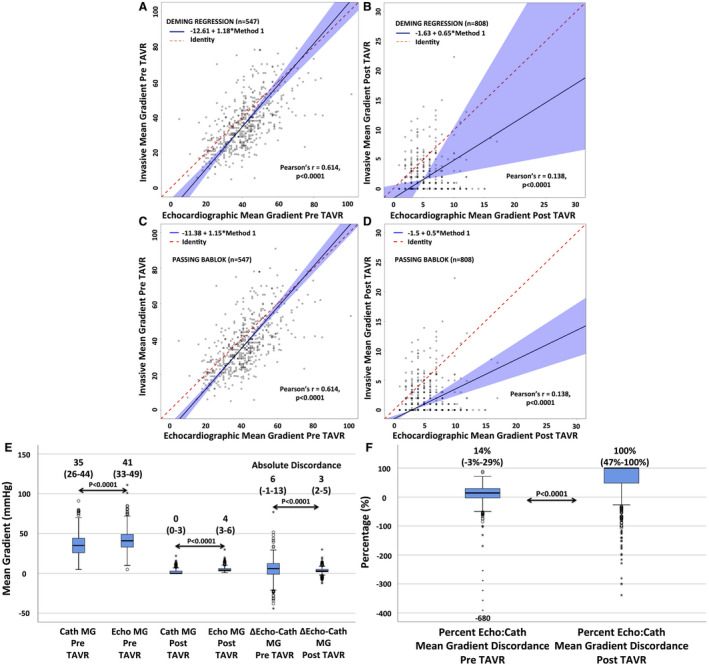
There is good correlation between Cath and Echo MGs in patients with AS (A) but a weak to no correlation (B) after TAVR, suggesting both modalities are not interchangeable. Echocardiography overestimates Cath MGs before and after TAVR (E). However, although there is a higher absolute discordance (Δ Echo‐Cath) between echocardiography and Cath MGs in AS compared with post‐TAVR (E), the percentage discordance (percentage Echo/Cath) is markedly higher post‐TAVR in a normal functioning prosthesis (100%) (F) than in AS (14%). Data presented as median (interquartile range).
Following TAVR, Deming and Passing‐Bablok regression reveal weak to no correlation (Pearson r=0.138; P<0.0001) (Figure 2B and 2E), and significant difference between echocardiographic (4 [3–6] mm Hg) versus invasive mean gradients (0 [0–3] mm Hg; P<0.0001) was noted (Figure 2E).
The absolute difference (absolute discordance) between echocardiographic and invasive mean gradients was higher pre‐TAVR (6 [−1 to 13] mm Hg) versus post‐TAVR (3 [2–5] mm Hg; P<0.0001) (Figure 2E), whereas the percentage difference (percentage discordance) was markedly lower pre‐TAVR (14% [−3% to 29%]) compared with post‐TAVR (100% [47%–100%]; P<0.0001) (Figure 2F).
BEV Versus SEV Invasive and Echocardiographic Mean Gradients and Discordance
Post‐TAVR, echocardiographic/invasive mean gradient absolute discordance was present in BEVs (4 [3–6] versus 0 [0–3] mm Hg, respectively; P<0.001) and SEVs (4 [3–7] versus 0 [0–2] mm Hg, respectively; P<0.0001).
Moreover, there was no significant difference between BEVs and SEVs in either their echocardiographic (P=0.967) or invasive mean gradients (P=0.167), and the absolute (3 [2–5] versus 3 [2–5] mm Hg, respectively; P=0.170) or percentage difference (100% [40%–100%] versus 100% [57%–100%], respectively; P=0.108) in discordance (Figure 3A).
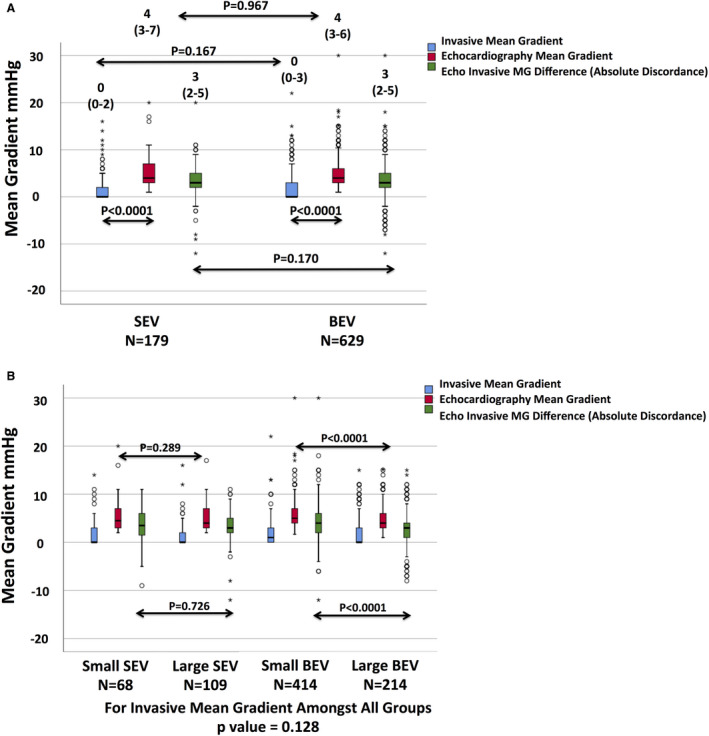
Data presented as median (interquartile range). Discordance between Echo and invasive MGs was present in both valves (A). There was no difference in Echo or invasive MGs, nor the difference between them in different platforms. Despite similar invasive MGs among all sizes, a small balloon‐expandable valve (BEV) demonstrated a higher Echo MG, a greater absolute discordance, and a similar percentage discordance between Echo and invasive MGs compared with a large BEV (B). Small and large self‐expanding valves (SEVs) exhibited similar Echo MGs, absolute discordance, and percentage discordance between Echo and invasive MGs.
Large Versus Small BEV and SEV Invasive and Echocardiographic Mean Gradients
Post‐TAVR, all valves, regardless of type or size, exhibited similar invasive mean gradients: 1 (0–3) mm Hg for small BEVs, 0 (0–3) mm Hg for large BEVs, 0 (0–3) mm Hg for small SEVs, and 0 (0–2) mm Hg for large SEVs (P=0.128) (Figure 3B).
However, small BEVs exhibited higher echocardiographic mean gradients (5 [4–7] mm Hg) compared with large BEVs (4 [3–6] mm Hg; P<0.0001). Conversely, small SEVs (4 [3–7] mm Hg) and large SEVs (4 [3–7] mm Hg) exhibited similar echocardiographic mean gradients (P=0.289) (Figure 3B). In a subanalysis of 20‐mm BEVs versus 23‐mm BEVs, 20‐mm BEVs exhibited even a higher echocardiographic mean gradient post‐TAVR compared with 23‐mm BEVs (8 [6–9] mm Hg versus 5 [4–7] mm Hg, respectively; P=0.003) despite a similar invasive gradient (P=0.544, for invasive mean gradients) (Figure S1).
In addition, small BEVs exhibited a greater absolute difference between echocardiographic and invasive mean gradients (4 [2–6] mm Hg) compared with large BEVs (3 [1–4] mm Hg; P<0.0001), whereas small and large SEVs exhibited similar absolute differences between their echocardiographic and invasive mean gradients (3 [2–6] mm Hg versus 3 [2–5] mm Hg, respectively; P=0.726) (Figure 3B).
The percentage difference between echocardiographic and invasive mean gradients was similar between small versus large SEVs (98.5% [43%–100%] versus 100% [67%–100%], respectively; P=0.062), and between small versus large BEVs (89.5% [50%–100%] versus 100% [40%–100%], respectively; P=0.734).
Post‐TAVR Versus Discharge Echocardiographic Hemodynamics
Discharge echocardiographic mean gradients were significantly higher than post‐TAVR (9 [7–12] mm Hg versus 4 [3–6] mm Hg, respectively; P<0.0001), in BEVs (10 [8–13] mm Hg versus 4 [3–6] mm Hg, respectively; P<0.0001) and in SEVs (7 [6–10] mm Hg versus 4 [3–7] mm Hg, respectively; P<0.0001).
Compared with SEVs, BEVs exhibited higher discharge mean gradient (P<0.0001), higher absolute difference (5 [3–7] mm Hg versus 3 [1–5] mm Hg, respectively; P<0.0001), and higher percentage difference between discharge and post‐TAVR echocardiographic mean gradients (120% [67%–200%] versus 67% [20%–125%], respectively; P<0.0001).
Small BEVs exhibited higher discharge mean gradients than any other valve type or size (Figure S2). The 20‐mm BEVs exhibited even a higher discharge mean gradient compared with 23‐mm BEVs (16 [12–21] mm Hg versus 11 [9–14] mm Hg, respectively; P=0.002) (Figure S2). Small BEVs exhibited a higher discharge mean gradient, higher absolute difference, but a similar percentage difference in discharge versus post‐TAVR mean gradients compared with larger BEVs, whereas small and large SEVs exhibited similar discharge mean gradients, absolute, and percentage differences (Figure S2 and Figure 4).
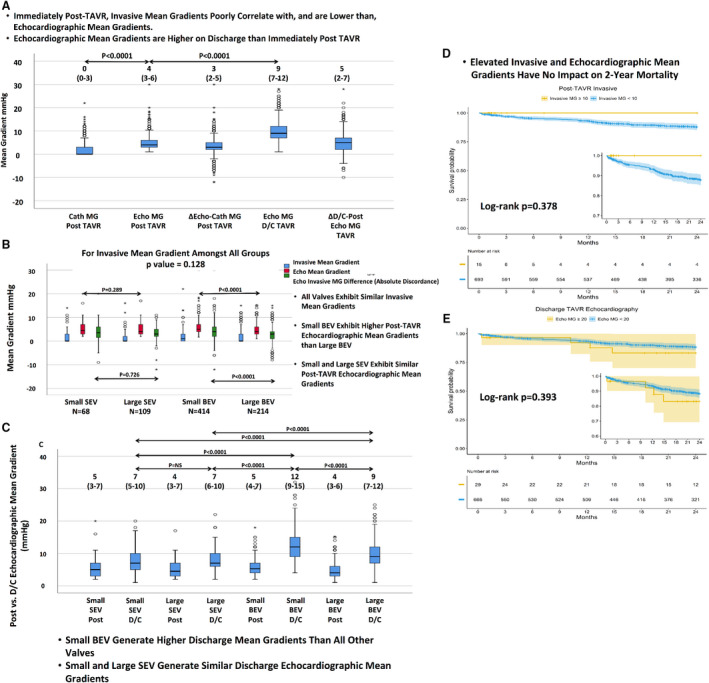
BEV indicates balloon‐expandable valve; Cath, invasive; D/C, discharge; Echo, echocardiographic; MG, mean gradient; SEV, self‐expanding valve; TAVR, transcatheter aortic valve replacement; ViV, valve in valve; Δ D/C‐post, difference between discharge and post‐Echo MG; and Δ Echo‐Cath, difference between echocardiography and catheterization MG (absolute discordance).
Invasive Versus Echocardiographic Mean Gradients and Mortality
The 30‐day mortality was 9 of 769 (1.1%), and the 2‐year mortality was 69 of 742 (9.2%). Neither an elevated post‐TAVR echocardiographic (log‐rank P=0.341) or invasive (log‐rank P=0.378) mean gradient ≥10 mm Hg nor an elevated discharge mean gradient ≥20 mm Hg (log‐rank P=0.393) was associated with increased 2‐year mortality. An increased Society of Thoracic Surgeons predicted risk of mortality (log‐rank P=0.007) and an ejection fraction <50% following TAVR (log‐rank P=0.028) were associated with increased 2‐year mortality (Figure 5).
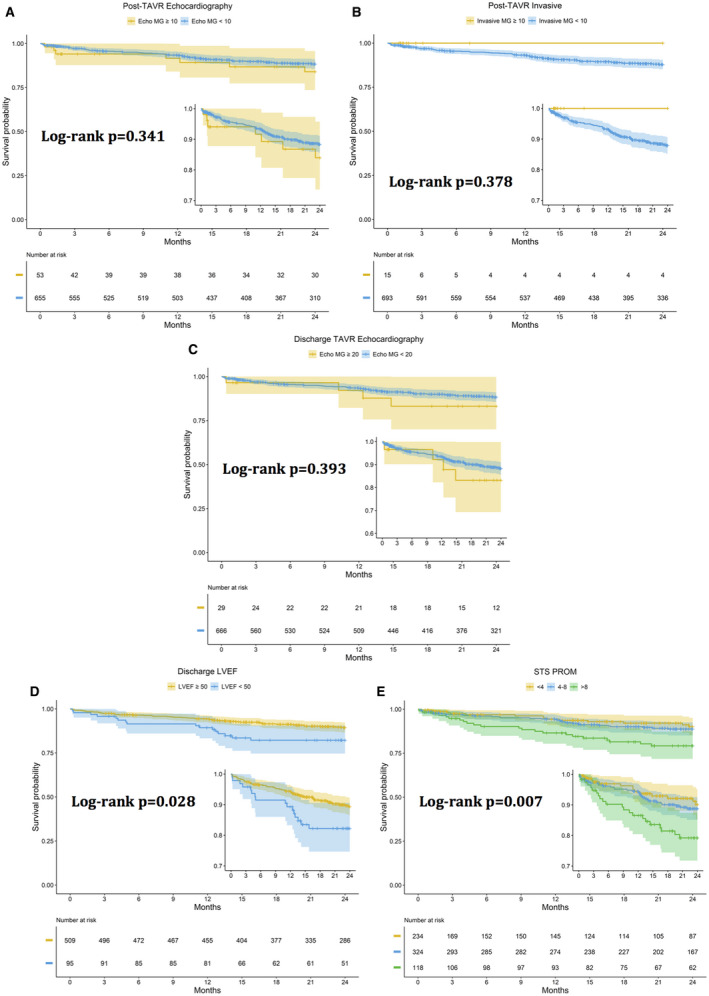
Increased STS PROM and LVEF <50%, but not elevated MGs, were associated with increased 2‐year mortality.
Discussion
This study demonstrates the following: (1) In contrast to patients with AS, there is weak to no correlation and agreement between echocardiography and invasive mean gradients following TAVR regardless of valve type or size. (2) Post‐TAVR, all valves sizes have similar invasive mean gradients. However, small BEVs (especially 20‐mm BEVs) exhibit a higher echocardiographic mean gradient compared with large BEVs, whereas small and large SEVs exhibit similar echocardiographic mean gradients. (3) Discharge echocardiographic mean gradients are significantly higher than post‐TAVR, significantly higher in BEVs compared with SEVs, and higher in small BEVs compared with all other valves. (4) Both BEVs and SEVs have overall excellent noninvasive and invasive hemodynamic profiles, low percentages of elevated mean gradients immediately post‐TAVR and at discharge, and remarkable clinical outcomes at 30 days and 2 years. Elevated echocardiographic or invasive mean gradients are not associated with increased 2‐year mortality.
We have previously reported post‐TAVR echocardiography/catheterization discordance following both native and valve‐in‐valve TAVR 22 , 23 , 28 in a single‐center analysis. Our current study confirms our previous findings and confirms that echocardiography‐derived transvalvular mean gradients in severe native AS correlate and compare well with invasive mean gradients. However, post‐TAVR, because of poor correlation and comparison of echocardiography‐derived mean gradients to direct‐invasive mean gradients, they are discordant and cannot be used interchangeably.
Discordance Between Echocardiographic and Invasive Mean Gradients
Discordance between echocardiographic and invasive mean gradients in AS and surgical prostheses is well described. 16 Following surgical aortic valve replacement, discordance is dependent on valve type and size, 18 , 21 , 24 is more prevalent in normally functioning compared with stenotic valves, 29 and cannot simply be explained by pressure recovery. 24 Similarly, post‐TAVR, adjusting for pressure recovery and the simplification of the Bernoulli equation improved, but did not resolve, discordance, especially after valve‐in‐valve TAVR. This potentially points to an inherent limitation of the Bernoulli equation when applied in normal functioning prosthesis beyond the effects of pressure recovery. 28
Although invasive gradient is directly measured (and not immune to measurement errors), the aortic valve velocity obtained by echocardiography is used in the Bernoulli equation to derive the mean gradient and in the continuity equation to derive the aortic valve area. The assumption is that the aortic velocity is only a reflection of the true valve gradient from a reduced area and vice versa. In reality, the reported echocardiographic mean gradient derived from the aortic valve velocity accounts only for, and attributes all increased velocity to, convective acceleration that is assumed to be from a reduced area. In addition to omitting the proximal LVOT pressure, Bernoulli omits other causes of increased velocity as nonconvective variables (flow acceleration and viscous forces), pressure recovery, flow amount, and type between different valves 30 , 31 , 32 (laminar versus turbulent, single versus multiple zones of flow convergence in series, and long versus short valve frames). An elevated aortic velocity attributable to the aforementioned factors, apart from a true transaortic gradient from a reduced area, is attributed by echocardiography as convective acceleration and thus leads to an unpredictable overestimation of the mean gradient. Although these effects occur in AS, their relative contribution is minor, because the increased velocity is primarily accounted for by the stenotic valve and thus the mean gradient is mainly channeled through convective acceleration. In nonstenotic prostheses, the simplification of Bernoulli, neglecting pressure recovery, and ignoring the Bernoulli equation requirements become more apparent, and their relative significance increases. 33
Although the numerical differences between invasive and echocardiographic mean gradients may be small post‐TAVR, there are several important points to consider. First, this is likely related to the population studied “post‐TAVR for native AS” only. We have previously demonstrated a larger absolute difference following valve‐in‐valve TAVR. 28 Second, the absolute difference between echocardiographic and invasive mean gradients is related to valve size and type; it is as low as 4 mm Hg for large BEVs and SEVs and as high as 8 mm Hg in the 20‐mm BEVs (Figure S1). Most patients had a large valve size, decreasing the absolute numerical difference. Third, even though the absolute discordance is small, the percentage discordance is elevated up to 100%, markedly higher compared with <15% in aortic stenosis (Figure 2F). Finally, the poor correlation between both modalities suggests they cannot be used interchangeably as a specific echocardiographic mean gradient may exhibit different invasive mean gradient values.
Discharge Versus Post‐TAVR Hemodynamics
Previous studies have reported lower mean gradients in AS under the effect of sedation and anesthesia. 34 Our study extends these findings to mean gradients post‐TAVR. Discharge mean gradients may be higher because of resolution of the effects of sedation, better patients' position and alignment of Doppler signal, and increased transvalvular flow. Although this phenomenon occurs in both valves, it is exaggerated in BEVs (120% increase in mean gradient), particularly small valves, compared with SEVs (<70% increase in mean gradient). The reason for this is unclear; however, differences in flow pattern between valves as well as the response to increased flow in different valve designs may be factors. Discharge mean gradients are the values reported to transcatheter valve therapy (TVT) registry, and this may explain the higher mean gradients reported by BEVs compared with SEVs and possibly suggesting a larger numerical difference between invasive mean gradients and TVT‐reported echocardiographic mean gradients.
Clinical Implications
The implications of this study are clinically significant. First, intraprocedural post‐TAVR invasive evaluation may be required to confirm elevated echocardiographic mean gradients because echocardiography overestimates invasive mean gradients and both modalities cannot be used interchangeably. The comparative hemodynamic performance of TAVR valves should not be based solely on the discharge echocardiographic mean gradient.
Second, the value of echocardiography to evaluate transaortic mean gradients noninvasively in patients with AS may not extend to patients with nonstenotic and otherwise normal TAVR valves given the poor correlation to invasive mean gradients. However, echocardiography remains an invaluable tool to follow up on changes from baseline mean gradients and grading of aortic regurgitation (AR) following TAVR.
Third, this study confirms the lack of impact of echocardiographic and invasive mean gradients on 2‐year mortality and argues that an elevated discharge echocardiographic mean gradient ≥20 mm Hg should not be equated with procedure futility or suboptimal valve hemodynamics, unless confirmed with multimodality imaging or invasive hemodynamics.
Limitations
A core laboratory did not analyze the echocardiographic or invasive data that were determined at each institution. However, careful attention to the fidelity of measurements was conducted and confirmed with multiple windows for echocardiography and careful balancing and flushing of the transducer for invasive measures. Moreover, invasive and echocardiographic measurements were obtained under similar hemodynamic conditions.
Patients were supine for echocardiography, and underestimation of the gradient may still be a possibility given the patient's position. Thus, echocardiography and invasive discordance may be higher.
It is impractical to obtain invasive mean gradients during long‐term follow‐up after TAVR. Thus, echocardiography remains important in identifying any significant change in mean gradient from the post‐TAVR as well as follow‐up on AR with the understanding that discharge mean gradients are higher than post‐TAVR. Larger studies may be required to determine the impact of elevated mean gradients and are currently ongoing.
The 2‐year follow‐up may be too short to show an impact on mortality by elevated mean gradients; thus, our findings are hypothesis generating.
Conclusions
Although a good correlation exists between echocardiographic and invasive mean gradients in patients with native AS, there is low to no correlation between these mean gradients post‐TAVR, suggesting they cannot be used interchangeably. Both BEVs and SEVs have overall excellent noninvasive and invasive hemodynamic profiles, with low percentages of elevated mean gradients immediately post‐TAVR and at discharge. All valve platforms, regardless of type and size, demonstrate discordance between their echocardiographic and invasive mean gradients, exhibit the same invasive mean gradient, and generate higher discharge echocardiographic mean gradients compared with post‐TAVR. Although the echocardiographic mean gradients are similar in large and small SEVs post‐TAVR and on discharge, small BEVs exhibit higher post‐TAVR compared with large BEVs and higher discharge echocardiographic mean gradients compared with all other valves. Elevated echocardiographic‐derived mean gradients post‐TAVR should be assessed with caution and compared with direct‐invasive mean gradients during the procedure.
Sources of Funding
None.
Disclosures
Drs Abbas, Pibarot, and Almariah have received research grants and consulting fees from Edwards Life Sciences. Drs Pibarot and Almariah have received research grants from Medtronic. Drs Pilgrim and Kassas received grants from Boston Scientific and Pilgrim and received speaker fees from Biotronik; consultancy for HighLife SAS and proctor for Boston Scientific and Medtronic. Dr Okuno reports speaker fees from Abbott. The remaining authors have no disclosures to report.
Notes
(J Am Heart Assoc. 2021;10:e021014. DOI: 10.1161/JAHA.120.021014.) [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.021014
For Sources of Funding and Disclosures, see page 12.
See Editorial by Reddy et al.
References
Articles from Journal of the American Heart Association: Cardiovascular and Cerebrovascular Disease are provided here courtesy of Wiley
Full text links
Read article at publisher's site: https://doi.org/10.1161/jaha.120.021014
Read article for free, from open access legal sources, via Unpaywall:
https://www.ahajournals.org/doi/pdf/10.1161/JAHA.120.021014
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/114260008
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1161/jaha.120.021014
Article citations
Clinical Implications of Good Post-TAVR Echocardiographic Hemodynamics: Do We Know Enough?
JACC Asia, 4(7):545-546, 08 Jul 2024
Cited by: 0 articles | PMID: 39101113 | PMCID: PMC11291384
Survival outcomes of TAVR and self-expanding versus balloon-expandable valves in patients with advanced cardiac dysfunction.
ESC Heart Fail, 11(3):1452-1462, 06 Feb 2024
Cited by: 2 articles | PMID: 38318998 | PMCID: PMC11098624
Differences in blood flow dynamics between balloon- and self-expandable valves in patients with aortic stenosis undergoing transcatheter aortic valve replacement.
J Cardiovasc Magn Reson, 25(1):60, 26 Oct 2023
Cited by: 0 articles | PMID: 37880721 | PMCID: PMC10601149
Long-term outcomes of measured and predicted prosthesis-patient mismatch following transcatheter aortic valve replacement.
EuroIntervention, 19(9):746-756, 01 Nov 2023
Cited by: 3 articles | PMID: 37622754 | PMCID: PMC10654767
Prosthesis-patient mismatch after transcatheter aortic valve implantation.
Cardiovasc Interv Ther, 37(4):615-625, 16 Jun 2022
Cited by: 3 articles | PMID: 35708855
Review
Go to all (12) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Echocardiographic Versus Invasive Aortic Valve Gradients in Different Clinical Scenarios.
J Am Soc Echocardiogr, 36(12):1302-1314, 26 Jul 2023
Cited by: 2 articles | PMID: 37507058
Transvalvular Pressure Gradients and All-Cause Mortality Following TAVR: A Multicenter Echocardiographic and Invasive Registry.
JACC Cardiovasc Interv, 15(18):1837-1848, 01 Sep 2022
Cited by: 4 articles | PMID: 36137687
Echocardiographic Results of Transcatheter Versus Surgical Aortic Valve Replacement in Low-Risk Patients: The PARTNER 3 Trial.
Circulation, 141(19):1527-1537, 10 Apr 2020
Cited by: 35 articles | PMID: 32272848
Recommendations for comprehensive intraprocedural echocardiographic imaging during TAVR.
JACC Cardiovasc Imaging, 8(3):261-287, 01 Mar 2015
Cited by: 42 articles | PMID: 25772834
Review

 1
,
2
1
,
2



