Abstract
Free full text

Spotlight on non-motor symptoms and Covid-19
Abstract
The Coronavirus Disease 2019 (Covid-19) pandemic has profoundly affected the quality of life (QoL) and health of the general population globally over the past 2 years, with a clear impact on people with Parkinson's Disease (PwP, PD). Non-motor symptoms have been widely acknowledged to hold a vital part in the clinical spectrum of PD, and, although often underrecognized, they significantly contribute to patients' and their caregivers' QoL. Up to now, there have been numerous reports of newly emerging or acutely deteriorating non-motor symptoms in PwP who had been infected by the Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2), while some of these symptoms, like fatigue, pain, depression, anxiety and cognitive impairment, have also been identified as part of the long-COVID syndrome due to their persistent nature. The subjacent mechanisms, mediating the appearance or progression of non-motor symptoms in the context of Covid-19, although probably multifactorial in origin, remain largely unknown. Such mechanisms might be, at least partly, related solely to the viral infection per se or the lifestyle changes imposed during the pandemic, as many of the non-motor symptoms seem to be prevalent even among Covid-19 patients without PD. Here, we summarize the available evidence and implications of Covid-19 in non-motor PD symptoms in the acute and chronic, if applicable, phase of the infection, with a special reference on studies of PwP.
1. Introduction
The Coronavirus Disease 2019 (Covid-19), caused by the Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2), has profoundly affected the global morbidity and mortality during the past 2 years with the survival of older individuals and those with chronic conditions, being particularly vulnerable (Abate, Checkol, & Mantefardo, 2021; Cascella, Rajnik, Aleem, Dulebohn, & Di Napoli, 2021). Researchers have been increasingly exploring the interaction of SARS-CoV-2 with pre-existing conditions, including Parkinson's Disease (PD), as numerous studies have revealed newly acquired or a worsening of pre-existent symptoms among people with PD (PwP) after being diagnosed with Covid-19. It is now widely acknowledged that non-motor symptoms, although often underrecognized, constitute a vital part in the multifaceted clinical spectrum of PD, significantly contributing to patients' and caregivers' disability and quality of life (QoL) (Chaudhuri, Healy, & Schapira, 2006; Martinez-Martin, Rodriguez-Blazquez, Kurtis, & Chaudhuri, 2011). Many of these symptoms, including constipation, hyposmia, rapid eye movement (REM) behavior disorder (RBD), fatigue and depression, might even precede the emergence of motor symptoms in PD by decades (Chaudhuri, Yates, & Martinez-Martin, 2005).
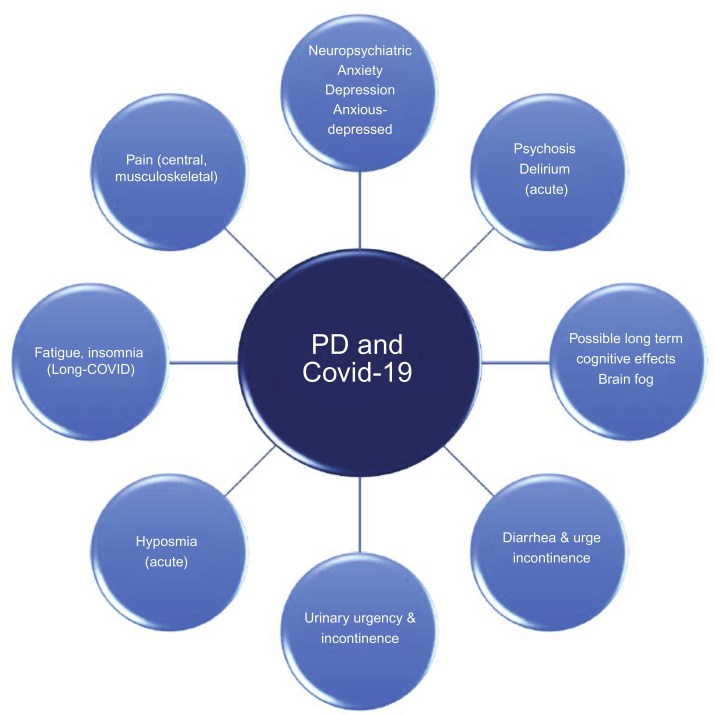
Our aim is to review and summarize the available evidence exploring the effect of Covid-19 on non-motor symptoms of PD both in the acute and, possibly, the chronic phase of the infection, as well as potential mechanisms that might mediate such effects on PwP (Fig. 1 ).
2. Depression
Depression has classically been known to correlate with social isolation among PwP (Karlsen, Larsen, Tandberg, & Maeland, 1999). Socially isolated caregivers of PwP have also experienced loneliness, leading to depression (McRae et al., 2009), which is even more critical in the current setting of the Covid-19 pandemic (Subramanian, Farahnik, & Mischley, 2020). Studies have shown that non-motor symptoms of PD, including mood changes, have generally deteriorated during the pandemic era, presenting either as part of the Covid-19 symptomatology (Hainque & Grabli, 2020) or in the course of the illness per se (Antonini, Leta, Teo, & Chaudhuri, 2020; Xia et al., 2020), leading to decreased QoL during this period (Suzuki et al., 2021). In the online study Fox Insight, a large cohort of adults with and without PD (5429 and 1452, respectively), 71% of PwP infected with SARS-CoV-2 have experienced new-onset or worsening of pre-existing depression, while the corresponding percentage for the non-infected PwP was 36.5% (Brown et al., 2020). Interestingly, the PwP mostly affected were usually women, irrespective of their Covid-19 status, even when social factors were accounted for (Brown et al., 2020; Picillo et al., 2021; Suzuki et al., 2021; Xia et al., 2020). Among those diagnosed with Covid-19, mood outcomes have been largely similar between people with and without PD (Brown et al., 2020; Suzuki et al., 2021). In a multicenter series of 27 PwP, depression has also been identified as part of the long-COVID syndrome, the entity used to describe the long-term sequelae of Covid-19 (Covid-19-associated symptoms persisting for over 12 weeks, without another possible explanation), affecting about 7.4% of the patients (Leta et al., 2021). Moreover, a recent large longitudinal general population-based cohort, exploring the rates of suicide among PwP (Chen et al., 2021) over 11 years, has indicated that suicide risk was two times higher in PwP compared to the general population, similarly to earlier studies (Eliasen, Dalhoff, & Horwitz, 2018; Erlangsen et al., 2020; Li et al., 2018), with late-life suicide rates being particularly high in East Asia. PD severity and co-morbid depression have been highlighted as the main correlates.
Factors associated with mood decline throughout the pandemic, include interruptions in medical care delivery, in essential daily activities and in regular exercise, loss of social contacts/activities, and/or self-isolation/quarantine (Brown et al., 2020; Kitani-Morii et al., 2021; van der Heide, Meinders, Bloem, & Helmich, 2020). A cross-sectional survey (n = 156) in Brazil has shown that lower physical activity level in PwP was associated with increased thoughts of death (Haas et al., 2022). Although the effect of the Covid-19 pandemic on mood changes is still unclear, increased awareness and screening for depression is strongly suggested among PwP, as the added impact of social distancing, limitations in exercise and PD-related complications might render patients particularly vulnerable.
3. Anxiety
It is widely acknowledged that PwP are at high risk of suffering from anxiety (Broen, Narayen, Kuijf, Dissanayaka, & Leentjens, 2016), and may be particularly susceptible to external stressors (Helmich & Bloem, 2020; van Wamelen, Wan, Ray Chaudhuri, & Jenner, 2020). Symptoms of both PD and anxiety, as well as the stress-related responses, have been thought to be involved in the cascade of dopaminergic, serotoninergic, and adrenergic pathways (Godoy, Rossignoli, Delfino-Pereira, Garcia-Cairasco, & de Lima Umeoka, 2018; Kano et al., 2011; van Wamelen et al., 2020).
It should, thus, come as no surprise that global converging evidence has indicated an increase in anxiety and stress among PwP infected with SARS-CoV-2 compared to healthy controls (Brown et al., 2020; Cilia et al., 2020; Shalash et al., 2020; Suzuki et al., 2021), although anxiety was the only significant predictor of worsening stress in PwP during the early days of the pandemic (Salari et al., 2020). A subjective worsening of anxiety, along with cognitive symptoms (with a mean Mini Mental State Examination score (MMSE) drop of 0.5 points), have been reported by 28 PwP in Pisa, Italy, throughout the course of lockdown (Palermo et al., 2020). In Rome, another study of 162 PwP has revealed that half of non-infected patients had experienced a worsening in their motor symptoms, with 25% of them reporting augmented anxiety during the pandemic (Schirinzi et al., 2020). A subsequent case-controlled survey in Tuscany has shown that 29.6% of non-infected patients in a PD cohort (n = 733) had experienced exacerbation of their motor symptoms with similar worsening of mood (24.7%), anxiety (25%), and poor sleep (22.2%) (Del Prete et al., 2021). An online survey exploring the impact of the pandemic on 358 PwP has shown that the pre-pandemic presence of neuropsychiatric symptoms, as well as cognitive dysfunction, was associated with increased levels of psychological distress within the pandemic (van der Heide et al., 2020).
Being home-bound and the loss of social contacts remained the commonest stressors among PwP (Zipprich, Teschner, Witte, Schonenberg, & Prell, 2020); others included reduced access to non-pharmacological treatments, such as physiotherapy and psychotherapy (Antonini et al., 2020; Song et al., 2020; Sulzer et al., 2020). Concurrent anxiety, stress, isolation, and physical inactivity have been found to be a particularly deleterious combination for PwP (Helmich & Bloem, 2020; Krzyszton et al., 2021).
In some countries with mandated lockdowns, the impact of anxiety among PwP appeared bleaker, with 25.5%–43.8% of them recording severe anxiety compared to controls, and more than half of PwP caregivers also reporting heightened anxiety (Oppo et al., 2020; Salari et al., 2020; Shalash et al., 2020). Specific contributing factors were mainly fear of the patient or the loved ones contracting SARS-CoV-2 (Krzyszton et al., 2021; Montanaro et al., 2022; Salari et al., 2020; Zipprich et al., 2020) and fear of limited drug availability (Guo et al., 2020; Montanaro et al., 2022; Salari et al., 2020; Shalash et al., 2020; Xia et al., 2020). In contrast, during the same time period in India, a telephone survey reported that patients and caregivers were “well-informed and coping well,” with only 11% of patients having experienced a worsening of their motor and non-motor symptoms (Prasad et al., 2020). This incongruity has been attributed to be due to the fact that considerably fewer people (n = 156) had died in India due to Covid-19 in the period immediately preceding the published article compared to other countries (World Health Organization, 2022).
A large, prospective cohort study had shown that anxiety levels before the pandemic, along with other motor and non-motor features, may predict future development of treatment-related motor complications (Kelly et al., 2019). This notion was reflected in a study taking place in March 2020 (De Micco et al., 2021), which revealed that the increased psychological impact of a 40-day quarantine in 94 PwP was significantly associated with a high pre-lockdown degree of anxiety, treatment-related PD motor complications, as well as the number of lockdown hours per day. PwP have reported markedly increased irritability, related to frequent thoughts of the lockdown, despite efforts not to think about it. There was no observed association noted between disease duration and severity with psychological well-being.
In a telephone survey of 100 individuals with advanced PD, Montanaro and colleagues have shown that anxiety and depression (as evaluated via the Hospital Anxiety and Depression Scale (HADS)) occurred in more than 30% of the participants and were significantly correlated with the type of PD treatment (Montanaro et al., 2022); patients on standard medical therapy and levodopa/carbidopa intestinal gel (LCIG) infusion exhibited the highest anxiety rates. Similarly to aforementioned studies (De Micco et al., 2021), no association was found with disease duration. The study also demonstrated that 40% and 21.7% of caregivers experienced anxiety and depression respectively (Montanaro et al., 2022).
Contrary to the majority of studies stating that the Covid-19-related lifestyle changes have led to negative outcomes in PwP, there has been some evidence that reduced external demands and activity levels may have been a relief to those that constantly had to adapt to disease-related changes or experience social pressure, thus reducing stress levels (Corti et al., 2018; HØrmann Thomsen, Wallerstedt, Winge, & Bergquist, 2021). A recent study has shown that the immediate effects of the Covid-19 period may have not been as disruptive as expected, with patients ratings and free-text descriptions suggesting that, despite the admittedly increased anxiety, the pandemic and the related changes in society have been associated with improvement in health-related QoL, improved sleep, and an overall feeling that the “pressure” is gone (HØrmann Thomsen et al., 2021). Another study in Morocco has shown that a 6-week confinement had not significantly affected the overall anxiety and depression scores of PwP (n = 50). It has been postulated that the unexpected improvement in mood and anxiety scores of a few patients may have been due to the regular presence of family members and an increased family support during the lockdown (El Otmani et al., 2021). The discrepant findings could be due to disparities in the socio-cultural framework across the different countries. Emergent PD anxiety may be secondary to an amalgamation of environmental vulnerability, dysfunctional coping strategies, as well as predisposing personality traits (Corti et al., 2018), factors likely to come to fore within the context of a crisis, like the Covid-19 pandemic.
In this period, PwP with high perceived stress levels have experienced more anxiety, ruminations, and neuroticism, scoring lower on cognitive abilities, social support, trait resilience, optimism, and positive appraisal style (van der Heide et al., 2020). Similarly to depression, those affected by anxiety were more frequently women (Picillo et al., 2021; Suzuki et al., 2021). Understanding the coping mechanisms and determinants of resilience for PwP, which may serve as prognostic factors (Weems, Costa, Dehon, & Berman, 2004; Whitworth et al., 2013), may be crucial in surviving a crisis, such as the Covid-19 pandemic.
However, the lack of consistent data on the risk of PwP developing Covid-19 (Artusi et al., 2020) has perpetuated the feelings of doubts and uncertainty, hence, increasing the level of anxiety; indeed, recent studies have recommended the critical requisite of correct and consistent information during the outbreak (Montanaro et al., 2022; Schirinzi et al., 2020). This highlights the key need for interventional planning during the post-lockdown phase (e.g., telemedicine services), aiming at bolstering the resilience of PwP toward a rapid resolution of potential mental health issues, triggered from this particularly stressful experience (De Micco et al., 2021).
4. Cognitive impairment
Acute viral central nervous system (CNS) infections, including cytomegalovirus (CMV), Epstein-Barr virus (EBV), human immunodeficiency virus (HIV), herpes viruses, varicella zoster virus (VZV) and hepatitis C virus (HCV), have been suggested to contribute to the subsequent development of cognitive impairment, even in previously healthy individuals, either via a direct viral insult of the nervous system or indirectly through promoting inflammation, epigenetic changes or a hypercoagulable state, which might alter brain structure and function (Damiano et al., 2021). An analysis of a large cohort of older individuals, who had been cognitively intact at baseline, has shown that acute care and critical or non-critical illness hospitalizations were associated with a higher risk of cognitive impairment during the following years (Ehlenbach et al., 2010). For older patients, who had been hospitalized due to acute systematic infections, a 1-year follow-up study has shown that premorbid dementia and delirium during hospitalization constituted risk factors for subsequent cognitive deterioration (Silva et al., 2021). Furthermore, a large recent cohort study of elderly nursing home residents has found that infection-related hospitalization was linked to an immediate and sustained cognitive decline, with older patients being more vulnerable (Gracner et al., 2021), while preliminary evidence supports that hospitalization per se due to an acute illness might precipitate cognitive impairment in older adults (Chinnappa-Quinn et al., 2020).
Recent data suggests that Covid-19, even when asymptomatic, might have both short- and long-term effects on patients' cognition (Damiano et al., 2021). A population-based study of 153 Covid-19 cases has revealed that 4% presented with a new-onset dementia-like syndrome during the acute phase of Covid-19 (Varatharaj et al., 2020). Small case series of Covid-19 patients, assessed using standardized neuropsychological tools, have revealed a decline in cognition detected about 2–4 weeks after infection with their attention being mostly impaired (Negrini et al., 2021; Zhou et al., 2020). In a cohort of 57 patients on rehabilitation after Covid-19 the vast majority has exhibited cognitive decline with attention and executive function being the most commonly affected domains (Jaywant et al., 2021). A large cross-sectional study in China has shown that Covid-19 was associated with long-term impairment in cognitive performance (assessment at 6 months) among patients older than 60, with severe Covid-19 and delirium being risk factors (Liu et al., 2021). A small sample size study has shown that younger Covid-19 patients with mild to moderate symptoms had persistent subclinical cognitive impairment, mostly affecting short-term memory, attention and concentration; these findings were not correlated to depressed mood or fatigue (Woo et al., 2020). Importantly, a recently published study, assessing brain changes in a sample of 785 UK Biobank participants (401 Covid-19-positive individuals and 384 controls), who had undergone a brain scan before and after being infected with SARS-CoV-2, has revealed a significant reduction in global brain size and in gray matter thickness and tissue-contrast in the orbitofrontal cortex and para-hippocampal gyrus, along with a decrease in the average cognitive performance before and after Covid-19 (Douaud et al., 2022). Although the authors could not conclude on the reversibility of these findings, they have highlighted the neurodegenerative potential of the virus.
A recent study assessing mice models and individuals with a mild SARS-CoV-2 infection has found a sustained elevation of CCL11, a circulating chemokine which has been associated with cognitive impairment and suspension of neurogenesis (Fernández-Castañeda et al., 2022). According to the authors, humans suffering from long-COVID, including cognitive symptoms, had higher levels of CCL11 compared to those with long-COVID, but without any cognitive complaints. What is more, affected mice have exhibited a persistent impairment in hippocampal neurogenesis, plus myelin loss in subcortical white matter. These pathophysiological changes might contribute to significant neurological sequelae, even after mild Covid-19.
Cognitive dysfunction is quite prevalent in PD with up to 83% of PwP presenting with some degree of cognitive impairment at some point of the disease course, usually at the advanced stages, and might be accompanied by psychosis and hallucinations (Schapira, Chaudhuri, & Jenner, 2017). However, mild or subclinical cognitive deficits, usually presenting as executive dysfunction or visuospatial impairment, can be diagnosed even in earlier PD stages (Aarsland et al., 2021). It is also of interest that, due to nigrostriatal dopamine depletion, PwP might experience cognitive inflexibility and decreased ability to cope with new circumstances, like the new daily routine imposed during the Covid-19 pandemic, a deficit that may also be associated with a sense of loss of control and psychological stress (Douma & de Kloet, 2020; Robbins & Cools, 2014). In the Covid-19 context, cognitive impairment might result either from direct disruption and damage of the virus on the CNS and the harmful sequalae (inflammation, hypercoagulation, hypoxia) or could be a secondary effect due to depression (Rock, Roiser, Riedel, & Blackwell, 2014), anxiety (Potvin, Hudon, Dion, Grenier, & Préville, 2011) or fatigue (Neu et al., 2011), as a result of the general psycho-social stress, isolation, social deprivation and routine changes accompanying the pandemic. Depression, anxiety and fatigue are also quite common non-motor symptoms in PD, and need to be considered and properly addressed whenever cognitive deficits are revealed (Schapira et al., 2017).
In a small, multicenter, 12-week study of 27 PwP with a Covid-19 diagnosis, new-onset cognitive disturbances appeared in 22.2% of patients, including “brain fog,” concentration impairment and memory deficits (Leta et al., 2021). In an online survey of 46 PwP and Covid-19, intellectual impairment has been detected in 48% of patients, although in this occasion no previous examinations had been available (Xu et al., 2021). In one community-based case-control study comparing PwP with and without Covid-19, cognitive performance has only been marginally affected (Cilia et al., 2020). In a group of 10 advanced PwP with Covid-19, cognition has been found to worsen during the infection (Antonini et al., 2020). In an Italian Movement Disorders Outpatient Clinic, 28 PwP have been diagnosed with new-onset cognitive disturbances and higher anxiety scores after the lockdown period, without being previously infected by SARS-CoV-2, thus, highlighting the effect of isolation on cognition (Palermo et al., 2020). However, these results have not been confirmed in a group of mild to moderate PD severity (Luis-Martínez et al., 2021). Researchers have attributed these new-onset clinical phenomena to a combination of factors, including the negative impact of the prolonged lockdown, the limited access to healthcare and rehabilitation services, the effect of the infection per se and the natural course of the disease. However, most of these studies have significant methodological flaws, like small sample sizes, lack of a control group or baseline assessments, so their results should be examined with caution. Interestingly, in a phone-based study of 568 Spanish PwP, it has been found that patients who had not reported a prior Covid-19 diagnosis were more likely to experience motor fluctuations (61% vs 35.7%, P = 0.052) and hallucinations (23.4% vs 0%, P = 0.025), and there was a tendency towards cognitive impairment and behavioral problems compared to those who had contracted a SARS-CoV-2 infection (Santos-García et al., 2020). The authors have suggested that stricter prevention measures, that might have prevented PwP from contracting Covid-19, might have also led to the above complications. However, the Covid-19-positive group in the above PD cohort was relatively small (2.6% of the total sample).
5. Psychosis
Psychosis is encountered in up to 40% of PwP, usually later in the disease course and typically manifests gradually, allowing for step-by-step approaches (Schapira et al., 2017; Simonet, Tolosa, Camara, & Valldeoriola, 2020). All dopaminergic medication can induce psychosis; levodopa in a lesser degree (Simonet et al., 2020). On the other hand, psychotic features can emerge secondarily due to other medication, like antidepressants, pain-killers or steroids, alcohol abuse or withdrawal, systematic or CNS infections, including Covid-19, CNS lesions, acute vascular events, or they can be part of the clinical spectrum of delirium, especially in elderly hospitalized patients (Dubovsky, Arvikar, Stern, & Axelrod, 2012; Parra et al., 2020; Saldanha, Menon, Chaudari, Bhattacharya, & Guliani, 2013; Webster & Holroyd, 2000). In the Covid-19 context, incident psychosis has not been very common, although more findings have been based on case reports and case series. According to a recent review of December 2021 summarizing the known published cases, delusions were the most typical sign of new-onset Covid-19 psychosis, and most patients had a favorable outcome, although 69% required hospitalization and 33% had to be admitted in a psychiatric facility (Smith et al., 2021). However, concurrent delirium was not excluded in the majority of cases and the possibility of bias was considered high in almost one out of three patients, either due to a history of substance abuse, psychiatric disorders or other medical conditions, which might be related to secondary psychosis (Smith et al., 2021). In the case-series of 10 advanced PwP diagnosed with Covid-19, no new-onset cases of psychosis was described, but psychotic features, which had been present prior to Covid-19, were reported to worsen during the infection (Antonini et al., 2020).
6. Delirium
Of note, delirium, an often life-threatening emergency, constitutes a common complication of Covid-19, especially in critically ill patients, and has been associated with a poor outcome (Ticinesi et al., 2020). Psychotic symptoms have been found to appear in almost half of the afflicted patients (Paik, Ahn, Min, Park, & Kim, 2018; Webster & Holroyd, 2000). Hospitalization per se can be a risk factor for the elderly, as delirium has been found to complicate the clinical course of one-third of the older general medical patients (Marcantonio, 2017). For those under mechanical ventilation admitted in the ICU this percentage has been found to exceed 75% (Ely et al., 2004). Decreased functional status, cognitive impairment, depression and comorbidities are typical predisposing factors, while subjacent infections, pain, anemia, anesthesia and drugs might act as precipitating factors (Kalimisetty, Askar, Fay, & Khan, 2017; Marcantonio, 2017; Wakefield, 2002). For Covid-19 patients, lack of visitation due to safety regulations imposed by the healthcare systems has also been identified as a risk factor (Pun et al., 2021). In two small sample size case-series of PwP with Covid-19, delirium has been found in 7–10% of patients (Antonini et al., 2020; Leta et al., 2021). Evidence suggests that PwP are more vulnerable to delirium while admitted in the hospital and treating physicians should be vigilant to acknowledge and timely manage any early sings of agitation, confusion or cognitive deterioration (Vardy, Teodorczuk, & Yarnall, 2015).
7. Hyposmia
Olfactory dysfunction constitutes one of the most commonly encountered symptoms in Covid-19 (Guerrero et al., 2021), although the majority of patients are expected to recover within a month (D'Ascanio et al., 2021). It is so typically connected to Covid-19, that a recent meta-analysis has shown it could be used as a good predictor of a SARS-CoV-2 infection (Hariyanto, Rizki, & Kurniawan, 2021). The olfactory epithelium is rich in common putative molecular site entries for SARS-CoV-2, like the Angiotensin Converting Enzyme-2 (ACE2) and Neuropilin 1 (NRP1), while the olfactory bulb is not protected by the blood–brain barrier (BBB), constituting theoretically an easier target for SARS-CoV-2 or other airborne viruses (Cantuti-Castelvetri et al., 2020; Wan et al., 2021). Reverse-transcription polymerase chain reaction (RT-PCR), in situ hybridization and immunohistochemical staining techniques have, indeed, revealed an increased SARS-CoV-2 viral load in the nasal epithelium (Meinhardt et al., 2021).
Impaired sense of smell is a highly prevalent non-motor symptom in PD as well, appearing in approximately 90% of PwP in the early or premotor prodromal stage, occasionally preceding the emergence of motor symptoms by years (Haehner, Hummel, & Reichmann, 2011; Xiao, Chen, & Le, 2014). Hyposmic PwP have been found to exhibit worse motor and cognitive progression, lower QoL scores and requiring higher levodopa equivalent doses (LED) compared to normosmic ones (Gjerde et al., 2018; He et al., 2020). The high percentage of olfactory dysfunction both in the PD and the Covid-19 population has recently given rise to concerns about SARS-CoV-2 infection being the “perfect storm” of a subsequent rise in parkinsonism cases (Brundin, Nath, & Beckham, 2020; Krey, Huber, Höglinger, & Wegner, 2021), although such notions remain highly speculative.
8. Gastrointestinal dysfunction
Gastrointestinal (GI) symptoms might affect up to three out of five patients with Covid-19 (Kariyawasam, Jayarajah, Riza, Abeysuriya, & Seneviratne, 2021). They are mostly mild and self-limited, and they might be prevalent even from the early stages of the infection (Kariyawasam et al., 2021; Villapol, 2020). Common symptoms include diarrhea, affecting about 10.4–11.5% of Covid-19 patients, followed by nausea and vomiting, which range from 6.3% to 10.5%, but more generic manifestations like anorexia or abdominal discomfort are often present (Andrews, Cai, Rudd, & Sanger, 2021; D'Amico, Baumgart, Danese, & Peyrin-Biroulet, 2020; Silva et al., 2020). Although subjacent acute causes from the GI system, including inflammatory or ischemic conditions, should be excluded, SARS-CoV-2 per se has been considered to insult the digestive tract, either directly or via a local or systemic inflammatory response to the virus. More particular, ACE2 and Transmembrane Serine Protease 2 (TMPRSS2), which supposedly constitute major cell entry receptors for SARS-CoV-2 (Hoffmann et al., 2020), are highly expressed in the intestinal endothelial cells of ileum and colon (Kariyawasam et al., 2021), where the virus is thought to disrupt the intestinal mucosal barrier and promote inflammation (Ye, Wang, Zhang, Xu, & Shang, 2020). SARS-CoV-2 RNA has, indeed, been detected in biopsy specimens of esophagus, stomach, duodenum and rectum from Covid-19 patients with clinically confirmed GI symptoms (Lin et al., 2020), while histopathological studies have revealed varying degrees of degeneration, necrosis and inflammation of the GI mucosa in post-mortem Covid-19 cases (Deshmukh, Motwani, Kumar, Kumari, & Raza, 2021). All the above indications have fueled speculations that the GI tract is a potential entry site and target organ for the virus.
During the last two decades a growing body of evidence has highlighted the role of the GI system in PD (Menozzi, Macnaughtan, & Schapira, 2021). GI dysfunction is quite common among PwP and all levels of the digestive tract can be affected, causing a range of symptoms, such as drooling, dysphagia, nausea, reduced gastric motility, constipation and impaired defecation (Schapira et al., 2017). The pathology underlying these manifestations is not quite clear yet, although α-synuclein aggregates, the pathological trademark of PD (Poewe et al., 2017), have been detected in the myenteric and submucosal plexuses of the enteric nervous system with a distinct rostro-caudal pattern of deposition (Fasano, Visanji, Liu, Lang, & Pfeiffer, 2015). These neurons originate centrally in the dorsal motor nucleus of the vagus nerve, which is also anatomically connected to the respiratory system. This has led researchers to assume that the dorsal vagal complex of the brainstem, which is highly enriched in ACE2, can be reached by SARS-CoV-2 through the vagus nerve from the periphery, allowing an entrance of the virus to the CNS (Rangon, Krantic, Moyse, & Fougère, 2020). Past experiments in mice have confirmed the transvagal transmission of Influenza A virus from the respiratory mucosa to the basal ganglia (Matsuda et al., 2004), while researchers have confirmed the potential interaction of the SARS-CoV-2 proteins with the intestine microstructure based on mice models (Mönkemüller, Fry, & Rickes, 2020). Interestingly, according to the dual-hit hypothesis of PD pathogenesis, a neurotropic pathogen, like a virus, has been speculated to enter the CNS through the nasal or gastric pathway, both of which appear to constitute sites of early pathology in PD (Hawkes, Del Tredici, & Braak, 2007; Klingelhoefer & Reichmann, 2015).
The emergence of GI symptoms in PwP inflicted by SARS-CoV-2 can overlap with already existing non-motor symptoms of the PD spectrum, complicating the patients' management, causing distress and worsening their QoL (Lubomski, Davis, & Sue, 2020; Menozzi et al., 2021). Diarrhea or vomiting in the setting of Covid-19 might impair the pharmacokinetics of orally administered dopaminergic medication, leading to reduced absorption of the drug and, consequently, an increased frequency and duration of OFF episodes. In a community-based case-control study comparing PwP with and without Covid-19 no statistically significant changes have been found considering the GI domains of the Non-Motor Symptoms Scale (NMSS) (Cilia et al., 2020). However, diarrhea has been found to exert a devasting effect on the pharmacokinetics of dopamine replacement therapy (particularly levodopa) and has been linked to an aggravation of motor PD symptoms (Cilia et al., 2020). Furthermore, dehydration due to diarrhea or reduced water intake in case of anorexia or vomiting, could precipitate or worsen already existent orthostatic hypotension, another common feature among PwP with dysautonomic features (Schapira et al., 2017; Simonet et al., 2020). Treating physicians should be aware that apart from SARS-CoV-2 per se, newly introduced medication in the context of Covid-19, like empirically used antibiotics, especially macrolides and quinolones, or the antiviral agent remdesivir, carry a significant risk of producing typical GI side effects, like nausea, vomiting, abdominal pain and diarrhea (Aleem & Kothadia, 2021; Patel & Hashmi, 2021; Yan & Bryant, 2021). Third-generation cephalosporines and quinolones have also been associated with a risk of pseudomembranous colitis, mediated by Clostridium difficile, a condition that requires special treatment and might further complicate the absorption and distribution of orally administered dopaminergic drugs (Arumugham, Gujarathi, & Cascella, 2021; Yan & Bryant, 2021).
Recent studies have suggested that patients with altered gut microbiota might experience more severe Covid-19 symptoms (Kim, 2021), while imbalances in the normal gut microbiota have been speculated to be involved in PD pathophysiology (Elfil, Kamel, Kandil, Koo, & Schaefer, 2020). A recent meta-analysis has confirmed consistent gut microbiome alterations among the population of PwP, which might be related to a pre-inflammatory status linked to the GI symptoms in PD (Romano et al., 2021). A small intestinal bacterial overgrowth has been observed in more than 60% of PwP and has been significantly associated with worse motor outcomes (Tan et al., 2014). Whether this parameter of gut dysbiosis might mediate part of the aggravation in motor and non-motor symptoms observed during PwP and Covid-19 needs to be further investigated.
Interestingly, recent reviews analyzing global data have shown that GI symptoms among Covid-19 survivors, including nausea, vomiting, diarrhea, anorexia, abdominal pain, acid reflux and constipation, might persist after their discharge or recovery (Silva Andrade et al., 2021; Yusuf et al., 2021). In a small, multicenter study with a small sample size of 27 PwP, followed for more than 12 weeks after a Covid-19 diagnosis, the prevalence of GI symptoms was low (7.4% for nausea and 3.7% for reduced appetite) (Leta et al., 2021). These prolonged manifestations, often referred to as long-COVID, might have an impact on patients' QoL and their pathogenesis remains to be clarified (Yusuf et al., 2021).
9. Dysautonomia
Orthostatic hypotension is an uncommon symptom in Covid-19 patients, with a frequency of 2.2%, being more prevalent in older individuals, those with concomitant high blood pressure and among beta blocker users (de Freitas et al., 2021). Despite the startling presentation, orthostatic hypotension has not been associated with a more severe outcome for Covid-19 (Oates et al., 2020).
Various pathophysiological mechanisms associated with Covid-19 and autonomic impairment have been described. The infection of the CNS nucleus tractus solitarius through the vagus nerve may affect the respiratory rhythm, heart rate regulation and blood pressure control. Likewise, the impairment of vagus nerve functioning, which regulates anti-inflammatory responses and the sympathetic overstimulation, may result in an increased release of pro-inflammatory cytokines (Del Rio, Marcus, & Inestrosa, 2020). Invasion of the vagus nucleus, specifically the nucleus ambiguous, could result in respiratory dysfunction, while excessive sympathetic activity can lead to cardiac arrhythmias and cathecolaminergic toxicity (Hassani, Fathi Jouzdani, Motarjem, Ranjbar, & Khansari, 2021). Postural orthostatic tachycardia syndrome (POTS), has been previously related to viral infections, such as influenza and EBV, and mainly appears as a post-infectious condition. In a case series of 20 patients with a prior Covid-19 diagnosis and evidence of orthostatic intolerance after examination (tilt table test), 75% has been diagnosed with POTS, which, interestingly, has persisted for 6–8 months after the infection. As POTS has been likely considered an autoimmune process, it is possible that SARS-CoV-2 might induce cross-reaction antibodies with autonomic related components, such as autonomic ganglia and nerve fibers, and cardiovascular receptors (Blitshteyn & Whitelaw, 2021; Díaz, Toledo, Andrade, Marcus, & Del Rio, 2020). Post-Covid-19 syndrome, as result of an autoimmune response after the initial SARS-CoV-2 infection, could be a trendsetting topic for future research with a possible benefit from inmunomodulatory therapies.
Early reports have suggested a higher frequency of orthostatic hypotension in PD and Covid-19 (Antonini et al., 2020), while the aforementioned online study Fox Insight has reported a worsening of dysautonomia in 38% of participants with Covid-19 and PD (Brown et al., 2020). Urge/incontinence, urine retention and nocturia in PD may be exacerbated after Covid-19 (Cilia et al., 2020; Xu et al., 2021); this phenomenon could be attributed to an increased consumption of over the counter drugs, such as antihistamine medication with anticholinergic properties used for Covid-19 mild symptoms, as they can aggravate constipation and urinary retention (Elbeddini, To, Tayefehchamani, & Wen, 2020). Motor fluctuations due to impaired absorption of dopaminergic drugs during Covid-19 could also have a deleterious impact on urinary symptoms (Cilia et al., 2020).
10. Pain
Pain is a common feature in both PD and Covid-19. It is present in around 40% of Covid-19 patients, with a higher prevalence in the female population (Lechien et al., 2020). In a survey of 46 PwP, who had been diagnosed with Covid-19 at the Columbia University Irving Medical Center, 40% described a worsening of pain and 4% noticed new-onset pain following the infection (Xu et al., 2021). In a similar trend, pain has been reported by 76% of PwP infected with SARS-CoV-2 in a cohort of 51 participants in the Fox Insight online study (Brown et al., 2020).
The mechanisms underlying the Covid-19-induced pain are still under investigation. Weng and colleagues have described different pathways associated with pain in Covid-19 (Weng, Su, & Wang, 2021). The systemic inflammation, which often accompanies Covid-19, implies an elevated release of cytokines, prostaglandins E1, E2 and bradykinin, and an up-regulation of interleukin-6 and could be associated with headache and polineuropathy, sore throat and myalgia. Likewise, elevated inflammatory markers may induce myocardial damage and retrosternal pain (Weng et al., 2021), while the increased ACE2 expression in the GI tract could potentially cause abdominal pain and diarrhea through mucosa inflammation (Lin et al., 2020).
The Covid-19 pandemic has had a negative impact on regular dopaminergic drugs intake, especially in non-white and low income individuals (Brown et al., 2020). Consequently, an increase in OFF periods and non-motor fluctuations, including pain, is expected (Rukavina et al., 2019). Systemic illness and inflammation could impair the pharmacodynamics of dopaminergic drugs and, therefore, newly induce or increase pre-existent pain. Finally, van der Heide and colleagues have found an aggravation of fatigue and pain in PwP, especially in women, as a result of psychological distress induced by the pandemic, while anxiety and increased rigidity might also have contributed (van der Heide et al., 2020). Different forms of pain have also been described as part of the long-COVID in PwP, including arthralgia (11.1%), muscular pain (7.4%), headache (18.5%) and peripheral neuropathy symptoms (11.1%). Less access to rehabilitation services and health centers during the pandemia, along with social isolation and worsening of motor and non-motor PD symptoms due to Covid-19, could be related to these long-term sequelae among PwP (Leta et al., 2021). Regular follow-up assessments of these patients could further enlight us about the impact of Covid-19 on the natural history of PD.
11. Fatigue
Fatigue is one of the most frequently reported symptoms among Covid-19 patients, affecting 38.1–63% of individuals with mild to moderate disease (Bhidayasiri, Virameteekul, Kim, Pal, & Chung, 2020; Lechien et al., 2020), while it usually persists for more than 12 weeks (El Sayed, Shokry, & Gomaa, 2021). Fatigue, whose differential diagnosis and management is at least challenging, constitutes a highly prevalent feature in PD with a negative impact on QoL of both patients and caregivers (Lazcano-Ocampo et al., 2020). Several studies have successively reported fatigue as one of the most frequent, either newly emerging or worsening, non-motor PD symptoms during a SARS-CoV-2 infection, particularly affecting women and elderly individuals (Cilia et al., 2020; El-Qushayri et al., 2021; Lechien et al., 2020). In a small cohort of 27 PwP and Covid-19 fatigue was one of the most prevalent symptom (47%) identified in the spectrum of long-COVID, along with motor worsening, cognitive impairment and sleep disturbances (Leta et al., 2021).
Fatigue in PD is a complex symptom with central and peripheral components. Central fatigue has been associated with cytokines released during the inflammatory response, affecting the basal ganglia and dopamine function (Felger & Miller, 2012). Moreover, it has been related to high levels of IL-6 and C-Reactive Protein (CRP) in the cerebrospinal fluid (CSF) and an elevated production of IL-1β (Lazcano-Ocampo et al., 2020). Interestingly, high levels of IL-6, IL-1β and CRP have also been detected in Covid-19 patients, as part of the inflammatory response to the virus, supporting the theory that elevated inflammatory interleukines might cause fatigue, not only in neurodegenerative diseases, but also in other cronic conditions (Sulzer et al., 2020). Physical fatigue has been found significantly higher in a cohort of Covid-19 patients in Turkey and has been associated with higher levels of CRP and lactate dehydrogenase (LDH) (Tuzun, Keles, Okutan, Yildiran, & Palamar, 2021). LDH has been linked to raised lactate levels in muscles, which might induce hypoxia and lead to muscle pain (Tuzun et al., 2021). Psychological distress and social isolation due to the pandemic have also been related with the exacerbation of some non-motor symptoms, such as fatigue and anxiety (van der Heide et al., 2020). Finally, many researchers would argue that fatigue could be entirely precipitated by the SARS-CoV-2 per se, and be fully irrelevant to non-motor PD symptoms in PD (Cilia et al., 2020).
12. Sleep impairment
The effect of the Covid-19 pandemic on sleep quality has not been unanimous among different studies and subpopulations. According to two recent systematic reviews and meta-analyses, with the larger one involving more than 345,000 participants from 39 countries, disruption of sleep appears to be quite prevalent during the ongoing pandemic with most of these problems having been associated with increased psychological distress, while patients with an active SARS-CoV-2 infection have been found particularly vulnerable (corrected pooled estimated prevalence of 18% in the general population and 57% among Covid-19 patients, P < 0.05) (Alimoradi et al., 2021; Jahrami et al., 2021). A great share of such disturbances of the sleep pattern have been attributed to the confinement regulations imposed during the pandemic. More specifically, a national cross-sectional study in Italy (n = 1515), the first European country applying a national lockdown for the Covid-19 pandemic, has revealed that 42.2% of the participants have been experiencing sleep problem during the pandemic, with 17.4% reporting a moderate to severe insomnia (Gualano, Lo Moro, Voglino, Bert, & Siliquini, 2020). In a telephone-based survey in Hawai, assessing 367 participants with chronic neurological conditions, including PD (3.5%), a percentage of 37.4% has reported sleep disturbances during the Covid-19 pandemic, irrespective of whether the responders had contracted SARS-CoV-2 or not (Crocker et al., 2022). On the other hand, researchers argue that the impact of Covid-19 on sleep patterns also depends on the pre-pandemic quality of sleep, with 25% of participants in an online survey in the Netherlands mentioning a meaningful amelioration in their sleep quality during the pandemic and 20% reporting the opposite (Kocevska, Blanken, Van Someren, & Rösler, 2020). According to the authors, the former group consisted mostly of individuals with pre-pandemic severe insomnia symptoms, while in the latter participants had mentioned no sleep complaints prior to the pandemic, thus, pointing out the highly heterogeneous nature of sleep problems. Such a trend has also been confirmed in a Danish/ Swedish cohort of 67 PwP with the majority of them (88%) reporting less sleep disturbances during the pandemic compared to the pre-Covid-19 period, leading the authors to suggest that the perceived alleviation in “pressure” and activity level might have been experienced as a relief for some patients, reflecting in their quality of sleep (HØrmann Thomsen et al., 2021).
Sleep impairment constitutes a prevalent feature in PD with a gradually rising frequency as the disease progresses (Schapira et al., 2017). PwP might be troubled by different kinds of sleep problems, which can be related both to the disease per se or the relative medication, and include insomnia, RBD, periodic limb movements, restless leg syndrome (RLS) and akathisia. A potential aggravation of motor symptoms, which can be expected in PwP during the pandemic, especially if they have contracted SARS-CoV-2 (Antonini et al., 2020; Brown et al., 2020; Cilia et al., 2020; Santos-García et al., 2020), has also been associated with exacerbations in sleep disturbances due to wearing off phenomena (Schapira et al., 2017). On the other hand, insomnia has been closely associated to depression and anxiety, which, as we have already mentioned, are quite prevalent both in PD and Covid-19, with those conditions often found to overlap and reinforce each other (Oh, Kim, Na, Cho, & Chu, 2019; Taylor, Lichstein, Durrence, Reidel, & Bush, 2005).
A cross-sectional, questionnaire-based survey in China, including 119 PwP and a control group of 169 sex- and age-matched healthy individuals, has shown that PwP had a higher prevalence of sleep problems (69.9% vs 44.4%, P < 0.001), which were independently associated to an aggravation of PD symptoms and anxiety, with female patients being particularly vulnerable (Xia et al., 2020). In an another cross-section, questionnaire-based study of PwP in India (n = 832), new-onset or an aggravation of pre-existing sleep problems has been mentioned by 23.9% of participants, with insomnia being the most prevalent issue (51.5%), followed by RLS worsening (24.7%) and RBD (22.7%) (Kumar et al., 2021). These problems, which have been related to worst QoL and an overall exacerbation of motor and non-motor symptoms, were more prevalent among those with advanced PD and those who had undergone longer periods of home isolation (> 60 days) or have been lacking proper family support during confinement. Physical activity and new hobbies during the lockdown have been found to act as protective factors on sleep impairment. In the online study of Fox Insight 4.5% and 32% of Covid-19-negative PwP have reported new-onset or worsening of sleep problems respectively (including insomnia, fatigue, excessive sleepiness, RBD), with this trend being more potent among female patients or those who had experienced interruptions in their PD-related medical care (Brown et al., 2020). Moreover, in a multicenter, international case series of 27 PwP, 22% has developed sleep disturbances as part of the long-COVID syndrome, suggesting that long-term sleep-related sequelae might also be expected (Leta et al., 2021). An online questionnaire of 417 PwP in Canada, assessing the physical and mental wellbeing, the daily activities and their PD symptoms, has revealed an aggravation of sleep disorders among those residing in Quebec, but not in other areas, leading the authors to suggest that some subpopulations might be more sensitive to social isolation and changes in their routine (de Rus Jacquet et al., 2021). This localization of results might be related to distinct rates of contracting a SARS-CoV-2 infection and different socio-cultural characteristics among separate regions even in the same country. Conclusively, it seems that sleep problems might be encountered during the Covid-19 pandemic in the population of PwP, however, a significant portion of these disturbances might be mediated by indirect factors accompanying the pandemic, and not the SARS-CoV-2 infection per se. More focused studies with larger sample size are needed to further explore this association.
13. Conclusion
The Covid-19 pandemic has had a clear impact on PwP and non-motor symptoms have been specifically affected, as they are both disease-specific and circumstantial. The unfortunate drawback of Covid-19-related public health measures, such as the imposed lockdown and isolation regulations, has had negative effects related to confinement and physical inactivity, which have been found to lead to worse QoL, stress, anxiety and depression (Pantell et al., 2013; Shalash et al., 2020) and many patients have witnessed a worsening of both motor and non-motor symptoms (Fabbri et al., 2021; Helmich & Bloem, 2020). Awareness of this constellation of non-motor symptoms affected by Covid-19 in PwP is required to improve and personalize patients' management.
References
- Aarsland D., Batzu L., Halliday G.M., Geurtsen G.J., Ballard C., Ray Chaudhuri K., et al. Parkinson disease-associated cognitive impairment. Nature Reviews. Disease Primers. 2021;7(1):47. 10.1038/s41572-021-00280-3. [Abstract] [CrossRef] [Google Scholar]
- Abate S.M., Checkol Y.A., Mantefardo B. Global prevalence and determinants of mortality among patients with COVID-19: A systematic review and meta-analysis. Annals of Medicine and Surgery. 2021;64 10.1016/j.amsu.2021.102204. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Aleem A., Kothadia J.P. StatPearls. StatPearls Publishing; Treasure Island (FL): 2021. (Copyright © 2021, StatPearls Publishing LLC) [Google Scholar]
- Alimoradi Z., Broström A., Tsang H.W.H., Griffiths M.D., Haghayegh S., Ohayon M.M., et al. Sleep problems during COVID-19 pandemic and its' association to psychological distress: A systematic review and meta-analysis. EClinicalMedicine. 2021;36 10.1016/j.eclinm.2021.100916. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Andrews P.L.R., Cai W., Rudd J.A., Sanger G.J. COVID-19, nausea, and vomiting. Journal of Gastroenterology and Hepatology. 2021;36(3):646–656. 10.1111/jgh.15261. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Antonini A., Leta V., Teo J., Chaudhuri K.R. Outcome of Parkinson's disease patients affected by COVID-19. Movement Disorders: Official Journal of the Movement Disorder Society. 2020;35(6):905–908. 10.1002/mds.28104. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Artusi C.A., Romagnolo A., Imbalzano G., Marchet A., Zibetti M., Rizzone M.G., et al. COVID-19 in Parkinson's disease: Report on prevalence and outcome. Parkinsonism & Related Disorders. 2020;80:7–9. 10.1016/j.parkreldis.2020.09.008. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Arumugham V.B., Gujarathi R., Cascella M. StatPearls Publishing; Treasure Island (FL): 2021. (Copyright © 2021, StatPearls Publishing LLC) [Abstract] [Google Scholar]
- Bhidayasiri R., Virameteekul S., Kim J.M., Pal P.K., Chung S.J. COVID-19: An early review of its global impact and considerations for Parkinson's disease patient care. The Journal of Movement Disorders. 2020;13(2):105–114. 10.14802/jmd.20042. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Blitshteyn S., Whitelaw S. Postural orthostatic tachycardia syndrome (POTS) and other autonomic disorders after COVID-19 infection: A case series of 20 patients. Immunologic Research. 2021;69(2):205–211. 10.1007/s12026-021-09185-5. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Broen M.P., Narayen N.E., Kuijf M.L., Dissanayaka N.N., Leentjens A.F. Prevalence of anxiety in Parkinson's disease: A systematic review and meta-analysis. Movement Disorders. 2016;31(8):1125–1133. 10.1002/mds.26643. [Abstract] [CrossRef] [Google Scholar]
- Brown E.G., Chahine L.M., Goldman S.M., Korell M., Mann E., Kinel D.R., et al. The effect of the COVID-19 pandemic on people with Parkinson's disease. Journal of Parkinson's Disease. 2020;10(4):1365–1377. 10.3233/jpd-202249. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Brundin P., Nath A., Beckham J.D. Is COVID-19 a perfect storm for Parkinson's disease? Trends in Neurosciences. 2020;43(12):931–933. 10.1016/j.tins.2020.10.009. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Cantuti-Castelvetri L., Ojha R., Pedro L.D., Djannatian M., Franz J., Kuivanen S., et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science (New York, N.Y.) 2020;370(6518):856–860. 10.1126/science.abd2985. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Cascella M., Rajnik M., Aleem A., Dulebohn S.C., Di Napoli R. StatPearls. StatPearls Publishing; Treasure Island (FL): 2021. (Copyright © 2021, StatPearls Publishing LLC) [Abstract] [Google Scholar]
- Chaudhuri K.R., Healy D.G., Schapira A.H. Non-motor symptoms of Parkinson's disease: Diagnosis and management. Lancet Neurology. 2006;5(3):235–245. 10.1016/s1474-4422(06)70373-8. [Abstract] [CrossRef] [Google Scholar]
- Chaudhuri K.R., Yates L., Martinez-Martin P. The non-motor symptom complex of Parkinson's disease: A comprehensive assessment is essential. Current Neurology and Neuroscience Reports. 2005;5(4):275–283. 10.1007/s11910-005-0072-6. [Abstract] [CrossRef] [Google Scholar]
- Chen Y.-Y., Yu S., Hu Y.-H., Li C.-Y., Artaud F., Carcaillon-Bentata L., et al. Risk of suicide among patients with Parkinson disease. JAMA Psychiatry. 2021;78(3):293–301. 10.1001/jamapsychiatry.2020.4001. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Chinnappa-Quinn L., Makkar S.R., Bennett M., Lam B.C.P., Lo J.W., Kochan N.A., et al. Is hospitalization a risk factor for cognitive decline in older age adults? International Psychogeriatrics. 2020;1-18 10.1017/s1041610220001763. [Abstract] [CrossRef] [Google Scholar]
- Cilia R., Bonvegna S., Straccia G., Andreasi N.G., Elia A.E., Romito L.M., et al. Effects of COVID-19 on Parkinson's disease clinical features: A community-based case-control study. Movement Disorders. 2020;35(8):1287–1292. 10.1002/mds.28170. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Corti E.J., Johnson A.R., Gasson N., Bucks R.S., Thomas M.G., Loftus A.M. Factor structure of the ways of coping questionnaire in Parkinson's disease. Parkinsons Disease. 2018;2018:7128069. 10.1155/2018/7128069. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Crocker J., Liu K., Smith M., Nakamoto M., Mitchell C., Zhu E., et al. Early impact of the COVID-19 pandemic on outpatient neurologic Care in Hawai'i. Hawai'i Journal of Health & Social Welfare. 2022;81(1):6–12. (Retrieved from https://pubmed.ncbi.nlm.nih.gov/35028589. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8742305/) [Europe PMC free article] [Abstract] [Google Scholar]
- Damiano R.F., Guedes B.F., de Rocca C.C., de Pádua Serafim A., Castro L.H.M., Munhoz C.D., et al. Cognitive decline following acute viral infections: Literature review and projections for post-COVID-19. European Archives of Psychiatry and Clinical Neuroscience. 2021;1-16 10.1007/s00406-021-01286-4. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- D'Amico F., Baumgart D.C., Danese S., Peyrin-Biroulet L. Diarrhea during COVID-19 infection: Pathogenesis, epidemiology, prevention, and management. Clinical Gastroenterology and Hepatology. 2020;18(8):1663–1672. 10.1016/j.cgh.2020.04.001. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- D'Ascanio L., Pandolfini M., Cingolani C., Latini G., Gradoni P., Capalbo M., et al. Olfactory dysfunction in COVID-19 patients: Prevalence and prognosis for recovering sense of smell. Otolaryngology and Head and Neck Surgery. 2021;164(1):82–86. 10.1177/0194599820943530. [Abstract] [CrossRef] [Google Scholar]
- de Freitas R.F., Torres S.C., Martín-Sánchez F.J., Carbó A.V., Lauria G., Nunes J.P.L. Syncope and COVID-19 disease - a systematic review. Autonomic Neuroscience. 2021;235 10.1016/j.autneu.2021.102872. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- De Micco R., Siciliano M., Sant'Elia V., Giordano A., Russo A., Tedeschi G., et al. Correlates of psychological distress in patients with Parkinson's disease during the COVID-19 outbreak. Movement Disorders Clinical Practice. 2021;8(1):60–68. 10.1002/mdc3.13108. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- de Rus Jacquet A., Bogard S., Normandeau C.P., Degroot C., Postuma R.B., Dupré N., et al. Clinical perception and management of Parkinson's disease during the COVID-19 pandemic: A Canadian experience. Parkinsonism & Related Disorders. 2021;91:66–76. 10.1016/j.parkreldis.2021.08.018. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Del Prete E., Francesconi A., Palermo G., Mazzucchi S., Frosini D., Morganti R., et al. Prevalence and impact of COVID-19 in Parkinson's disease: Evidence from a multi-center survey in Tuscany region. Journal of Neurology. 2021;268(4):1179–1187. 10.1007/s00415-020-10002-6. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Del Rio R., Marcus N.J., Inestrosa N.C. Potential role of autonomic dysfunction in Covid-19 morbidity and mortality. Frontiers in Physiology. 2020;11 10.3389/fphys.2020.561749. 561749. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Deshmukh V., Motwani R., Kumar A., Kumari C., Raza K. Histopathological observations in COVID-19: A systematic review. Journal of Clinical Pathology. 2021;74(2):76–83. 10.1136/jclinpath-2020-206995. [Abstract] [CrossRef] [Google Scholar]
- Díaz H.S., Toledo C., Andrade D.C., Marcus N.J., Del Rio R. Neuroinflammation in heart failure: New insights for an old disease. The Journal of Physiology. 2020;598(1):33–59. 10.1113/jp278864. [Abstract] [CrossRef] [Google Scholar]
- Douaud G., Lee S., Alfaro-Almagro F., Arthofer C., Wang C., McCarthy P., et al. SARS-CoV-2 is associated with changes in brain structure in UK biobank. Nature. 2022 10.1038/s41586-022-04569-5. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Douma E.H., de Kloet E.R. Stress-induced plasticity and functioning of ventral tegmental dopamine neurons. Neuroscience and Biobehavioral Reviews. 2020;108:48–77. 10.1016/j.neubiorev.2019.10.015. [Abstract] [CrossRef] [Google Scholar]
- Dubovsky A.N., Arvikar S., Stern T.A., Axelrod L. The neuropsychiatric complications of glucocorticoid use: Steroid psychosis revisited. Psychosomatics. 2012;53(2):103–115. 10.1016/j.psym.2011.12.007. [Abstract] [CrossRef] [Google Scholar]
- Ehlenbach W.J., Hough C.L., Crane P.K., Haneuse S.J., Carson S.S., Curtis J.R., et al. Association between acute care and critical illness hospitalization and cognitive function in older adults. JAMA. 2010;303(8):763–770. 10.1001/jama.2010.167. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- El Otmani H., El Bidaoui Z., Amzil R., Bellakhdar S., El Moutawakil B., Abdoh Rafai M. No impact of confinement during COVID-19 pandemic on anxiety and depression in parkinsonian patients. Revue Neurologique (Paris) 2021;177(3):272–274. 10.1016/j.neurol.2021.01.005. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- El Sayed S., Shokry D., Gomaa S.M. Post-COVID-19 fatigue and anhedonia: A cross-sectional study and their correlation to post-recovery period. Neuropsychopharmacology Reports. 2021;41(1):50–55. 10.1002/npr2.12154. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Elbeddini A., To A., Tayefehchamani Y., Wen C. Potential impact and challenges associated with Parkinson's disease patient care amidst the COVID-19 global pandemic. Journal of Clinical Movement Disorders. 2020;7:7. 10.1186/s40734-020-00089-4. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Elfil M., Kamel S., Kandil M., Koo B.B., Schaefer S.M. Implications of the gut microbiome in Parkinson's disease. Movement Disorders. 2020;35(6):921–933. 10.1002/mds.28004. [Abstract] [CrossRef] [Google Scholar]
- Eliasen A., Dalhoff K.P., Horwitz H. Neurological diseases and risk of suicide attempt: A case-control study. Journal of Neurology. 2018;265(6):1303–1309. 10.1007/s00415-018-8837-4. [Abstract] [CrossRef] [Google Scholar]
- El-Qushayri A.E., Ghozy S., Reda A., Kamel A.M.A., Abbas A.S., Dmytriw A.A. The impact of Parkinson's disease on manifestations and outcomes of Covid-19 patients: A systematic review and meta-analysis. Reviews in Medical Virology. 2021;e2278 10.1002/rmv.2278. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Ely E.W., Shintani A., Truman B., Speroff T., Gordon S.M., Harrell F.E., Jr., et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753–1762. 10.1001/jama.291.14.1753. [Abstract] [CrossRef] [Google Scholar]
- Erlangsen A., Stenager E., Conwell Y., Andersen P.K., Hawton K., Benros M.E., et al. Association between neurological disorders and death by suicide in Denmark. JAMA. 2020;323(5):444–454. 10.1001/jama.2019.21834. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Fabbri M., Leung C., Baille G., Béreau M., Brefel Courbon C., Castelnovo G., et al. A French survey on the lockdown consequences of COVID-19 pandemic in Parkinson's disease. The ERCOPARK study. Parkinsonism & Related Disorders. 2021;89:128–133. 10.1016/j.parkreldis.2021.07.013. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Fasano A., Visanji N.P., Liu L.W., Lang A.E., Pfeiffer R.F. Gastrointestinal dysfunction in Parkinson's disease. Lancet Neurology. 2015;14(6):625–639. 10.1016/s1474-4422(15)00007-1. [Abstract] [CrossRef] [Google Scholar]
- Felger J.C., Miller A.H. Cytokine effects on the basal ganglia and dopamine function: The subcortical source of inflammatory malaise. Frontiers in Neuroendocrinology. 2012;33(3):315–327. 10.1016/j.yfrne.2012.09.003. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Fernández-Castañeda A., Lu P., Geraghty A.C., Song E., Lee M.-H., Wood J., et al. Mild respiratory SARS-CoV-2 infection can cause multi-lineage cellular dysregulation and myelin loss in the brain. bioRxiv. 2022 10.1101/2022.01.07.475453. 2022.2001.2007.475453. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Gjerde K.V., Müller B., Skeie G.O., Assmus J., Alves G., Tysnes O.B. Hyposmia in a simple smell test is associated with accelerated cognitive decline in early Parkinson's disease. Acta Neurologica Scandinavica. 2018;138(6):508–514. 10.1111/ane.13003. [Abstract] [CrossRef] [Google Scholar]
- Godoy L.D., Rossignoli M.T., Delfino-Pereira P., Garcia-Cairasco N., de Lima Umeoka E.H. A comprehensive overview on stress neurobiology: Basic concepts and clinical implications. Frontiers in Behavioral Neuroscience. 2018;12:127. 10.3389/fnbeh.2018.00127. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Gracner T., Agarwal M., Murali K.P., Stone P.W., Larson E.L., Furuya E.Y., et al. Association of Infection-Related Hospitalization with Cognitive Impairment among Nursing Home Residents. JAMA Network Open. 2021;4(4) 10.1001/jamanetworkopen.2021.7528. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Gualano M.R., Lo Moro G., Voglino G., Bert F., Siliquini R. Effects of Covid-19 lockdown on mental health and sleep disturbances in Italy. International Journal of Environmental Research and Public Health. 2020;17(13) 10.3390/ijerph17134779. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Guerrero J.I., Barragán L.A., Martínez J.D., Montoya J.P., Peña A., Sobrino F.E., et al. Central and peripheral nervous system involvement by COVID-19: A systematic review of the pathophysiology, clinical manifestations, neuropathology, neuroimaging, electrophysiology, and cerebrospinal fluid findings. BMC Infectious Diseases. 2021;21(1):515. 10.1186/s12879-021-06185-6. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Guo D., Han B., Lu Y., Lv C., Fang X., Zhang Z., et al. Influence of the COVID-19 pandemic on quality of life of patients with Parkinson's disease. Parkinsons Disease. 2020;2020:1216568. 10.1155/2020/1216568. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Haas A.N., Passos-Monteiro E., Delabary M.D.S., Moratelli J., Schuch F.B., Correa C.L., et al. Association between mental health and physical activity levels in people with Parkinson's disease during the COVID-19 pandemic: An observational cross-sectional survey in Brazil. Sport Science for Health. 2022;1-7 10.1007/s11332-021-00868-y. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Haehner A., Hummel T., Reichmann H. Olfactory loss in Parkinson's disease. Parkinson's Disease. 2011;2011 10.4061/2011/450939. 450939. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Hainque E., Grabli D. Rapid worsening in Parkinson's disease may hide COVID-19 infection. Parkinsonism & Related Disorders. 2020;75:126–127. 10.1016/j.parkreldis.2020.05.008. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Hariyanto T.I., Rizki N.A., Kurniawan A. Anosmia/hyposmia is a good predictor of coronavirus disease 2019 (COVID-19) infection: A meta-analysis. International Archives of Otorhinolaryngology. 2021;25(1):e170–e174. 10.1055/s-0040-1719120. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Hassani M., Fathi Jouzdani A., Motarjem S., Ranjbar A., Khansari N. How COVID-19 can cause autonomic dysfunctions and postural orthostatic syndrome? A review of mechanisms and evidence. Neurology and Clinical Neuroscience. 2021;9(6):434–442. 10.1111/ncn3.12548. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Hawkes C.H., Del Tredici K., Braak H. Parkinson's disease: A dual-hit hypothesis. Neuropathology and Applied Neurobiology. 2007;33(6):599–614. 10.1111/j.1365-2990.2007.00874.x. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- He R., Zhao Y., He Y., Zhou Y., Yang J., Zhou X., et al. Olfactory dysfunction predicts disease progression in Parkinson's disease: A longitudinal study. Frontiers in Neuroscience. 2020;14 10.3389/fnins.2020.569777. 569777. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Helmich R.C., Bloem B.R. The impact of the COVID-19 pandemic on Parkinson's disease: Hidden sorrows and emerging opportunities. Journal of Parkinson's Disease. 2020;10(2):351–354. 10.3233/JPD-202038. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. 10.1016/j.cell.2020.02.052. e278. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- HØrmann Thomsen T., Wallerstedt S.M., Winge K., Bergquist F. Life with Parkinson's disease during the COVID-19 pandemic: The pressure is "OFF". Journal of Parkinson's Disease. 2021;11(2):491–495. 10.3233/jpd-202342. [Abstract] [CrossRef] [Google Scholar]
- Jahrami H., BaHammam A.S., Bragazzi N.L., Saif Z., Faris M., Vitiello M.V. Sleep problems during the COVID-19 pandemic by population: A systematic review and meta-analysis. Journal of Clinical Sleep Medicine. 2021;17(2):299–313. 10.5664/jcsm.8930. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Jaywant A., Vanderlind W.M., Alexopoulos G.S., Fridman C.B., Perlis R.H., Gunning F.M. Frequency and profile of objective cognitive deficits in hospitalized patients recovering from COVID-19. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2021;46(13):2235–2240. 10.1038/s41386-021-00978-8. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Kalimisetty S., Askar W., Fay B., Khan A. Models for predicting incident delirium in hospitalized older adults: A systematic review. Journal of Patient-Centered Research and Reviews. 2017;4(2):69–77. 10.17294/2330-0698.1414. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Kano O., Ikeda K., Cridebring D., Takazawa T., Yoshii Y., Iwasaki Y. Neurobiology of depression and anxiety in Parkinson's disease. Parkinsons Disease. 2011;2011 10.4061/2011/143547. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Kariyawasam J.C., Jayarajah U., Riza R., Abeysuriya V., Seneviratne S.L. Gastrointestinal manifestations in COVID-19. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2021;115(12):1362–1388. 10.1093/trstmh/trab042. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Karlsen K.H., Larsen J.P., Tandberg E., Maeland J.G. Influence of clinical and demographic variables on quality of life in patients with Parkinson's disease. Journal of Neurology, Neurosurgery, and Psychiatry. 1999;66(4):431–435. 10.1136/jnnp.66.4.431. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Kelly M.J., Lawton M.A., Baig F., Ruffmann C., Barber T.R., Lo C., et al. Predictors of motor complications in early Parkinson's disease: A prospective cohort study. Movement Disorders. 2019;34(8):1174–1183. 10.1002/mds.27783. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Kim H.S. Do an altered gut microbiota and an associated leaky gut affect COVID-19 severity? MBio. 2021;12(1) 10.1128/mBio.03022-20. e03022-03020. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Kitani-Morii F., Kasai T., Horiguchi G., Teramukai S., Ohmichi T., Shinomoto M., et al. Risk factors for neuropsychiatric symptoms in patients with Parkinson's disease during COVID-19 pandemic in Japan. PLoS One. 2021;16(1) 10.1371/journal.pone.0245864. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Klingelhoefer L., Reichmann H. Pathogenesis of Parkinson disease—The gut-brain axis and environmental factors. Nature Reviews. Neurology. 2015;11(11):625–636. 10.1038/nrneurol.2015.197. [Abstract] [CrossRef] [Google Scholar]
- Kocevska D., Blanken T.F., Van Someren E.J.W., Rösler L. Sleep quality during the COVID-19 pandemic: Not one size fits all. Sleep Medicine. 2020;76:86–88. 10.1016/j.sleep.2020.09.029. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Krey L., Huber M.K., Höglinger G.U., Wegner F. Can SARS-CoV-2 infection Lead to neurodegeneration and Parkinson's disease? Brain Sciences. 2021;11(12) 10.3390/brainsci11121654. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Krzyszton K., Mielanczuk-Lubecka B., Stolarski J., Poznanska A., Kepczynska K., Zdrowowicz A., et al. Secondary impact of COVID-19 pandemic on people with Parkinson's disease-results of a polish online survey. Brain Sciences. 2021;12(1) 10.3390/brainsci12010026. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Kumar N., Gupta R., Kumar H., Mehta S., Rajan R., Kumar D., et al. Impact of home confinement during COVID-19 pandemic on sleep parameters in Parkinson's disease. Sleep Medicine. 2021;77:15–22. 10.1016/j.sleep.2020.11.021. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Lazcano-Ocampo C., Wan Y.M., van Wamelen D.J., Batzu L., Boura I., Titova N., et al. Identifying and responding to fatigue and apathy in Parkinson's disease: A review of current practice. Expert Review of Neurotherapeutics. 2020;20(5):477–495. 10.1080/14737175.2020.1752669. [Abstract] [CrossRef] [Google Scholar]
- Lechien J.R., Chiesa-Estomba C.M., Place S., Van Laethem Y., Cabaraux P., Mat Q., et al. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. Journal of Internal Medicine. 2020;288(3):335–344. 10.1111/joim.13089. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Leta V., Rodríguez-Violante M., Abundes A., Rukavina K., Teo J.T., Falup-Pecurariu C., et al. Parkinson's disease and post-COVID-19 syndrome: The Parkinson's long-COVID Spectrum. Movement Disorders. 2021;36(6):1287–1289. 10.1002/mds.28622. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Li W., Abbas M.M., Acharyya S., Ng H.L., Tay K.Y., Au W.L., et al. Suicide in Parkinson's disease. Movement Disorders Clinical Practice. 2018;5(2):177–182. 10.1002/mdc3.12599. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Lin L., Jiang X., Zhang Z., Huang S., Zhang Z., Fang Z., et al. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69(6):997–1001. 10.1136/gutjnl-2020-321013. [Abstract] [CrossRef] [Google Scholar]
- Liu Y.H., Wang Y.R., Wang Q.H., Chen Y., Chen X., Li Y., et al. Post-infection cognitive impairments in a cohort of elderly patients with COVID-19. Molecular Neurodegeneration. 2021;16(1):48. 10.1186/s13024-021-00469-w. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Lubomski M., Davis R.L., Sue C.M. Gastrointestinal dysfunction in Parkinson's disease. Journal of Neurology. 2020;267(5):1377–1388. 10.1007/s00415-020-09723-5. [Abstract] [CrossRef] [Google Scholar]
- Luis-Martínez R., Di Marco R., Weis L., Cianci V., Pistonesi F., Baba A., et al. Impact of social and mobility restrictions in Parkinson's disease during COVID-19 lockdown. BMC Neurology. 2021;21(1):332. 10.1186/s12883-021-02364-9. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Marcantonio E.R. Delirium in Hospitalized Older Adults. The New England Journal of Medicine. 2017;377(15):1456–1466. 10.1056/NEJMcp1605501. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Martinez-Martin P., Rodriguez-Blazquez C., Kurtis M.M., Chaudhuri K.R. The impact of non-motor symptoms on health-related quality of life of patients with Parkinson's disease. Movement Disorders. 2011;26(3):399–406. 10.1002/mds.23462. [Abstract] [CrossRef] [Google Scholar]
- Matsuda K., Park C.H., Sunden Y., Kimura T., Ochiai K., Kida H., et al. The vagus nerve is one route of transneural invasion for intranasally inoculated influenza a virus in mice. Veterinary Pathology. 2004;41(2):101–107. 10.1354/vp.41-2-101. [Abstract] [CrossRef] [Google Scholar]
- McRae C., Fazio E., Hartsock G., Kelley L., Urbanski S., Russell D. Predictors of loneliness in caregivers of persons with Parkinson's disease. Parkinsonism & Related Disorders. 2009;15(8):554–557. 10.1016/j.parkreldis.2009.01.007. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Meinhardt J., Radke J., Dittmayer C., Franz J., Thomas C., Mothes R., et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nature Neuroscience. 2021;24(2):168–175. 10.1038/s41593-020-00758-5. [Abstract] [CrossRef] [Google Scholar]
- Menozzi E., Macnaughtan J., Schapira A.H.V. The gut-brain axis and Parkinson disease: Clinical and pathogenetic relevance. Annals of Medicine. 2021;53(1):611–625. 10.1080/07853890.2021.1890330. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Mönkemüller K., Fry L., Rickes S. COVID-19, coronavirus, SARS-CoV-2 and the small bowel. Revista Española de Enfermedades Digestivas. 2020;112(5):383–388. 10.17235/reed.2020.7137/2020. [Abstract] [CrossRef] [Google Scholar]
- Montanaro E., Artusi C.A., Rosano C., Boschetto C., Imbalzano G., Romagnolo A., et al. Anxiety, depression, and worries in advanced Parkinson disease during COVID-19 pandemic. Neurological Sciences. 2022;43(1):341–348. 10.1007/s10072-021-05286-z. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Negrini F., Ferrario I., Mazziotti D., Berchicci M., Bonazzi M., de Sire A., et al. Neuropsychological features of severe hospitalized coronavirus disease 2019 patients at clinical stability and clues for Postacute rehabilitation. Archives of Physical Medicine and Rehabilitation. 2021;102(1):155–158. 10.1016/j.apmr.2020.09.376. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Neu D., Kajosch H., Peigneux P., Verbanck P., Linkowski P., Le Bon O. Cognitive impairment in fatigue and sleepiness associated conditions. Psychiatry Research. 2011;189(1):128–134. 10.1016/j.psychres.2010.12.005. [Abstract] [CrossRef] [Google Scholar]
- Oates C.P., Turagam M.K., Musikantow D., Chu E., Shivamurthy P., Lampert J., et al. Syncope and presyncope in patients with COVID-19. Pacing and Clinical Electrophysiology. 2020;43(10):1139–1148. 10.1111/pace.14047. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Oh C.M., Kim H.Y., Na H.K., Cho K.H., Chu M.K. The effect of anxiety and depression on sleep quality of individuals with high risk for insomnia: A population-based study. Frontiers in Neurology. 2019;10:849. 10.3389/fneur.2019.00849. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Oppo V., Serra G., Fenu G., Murgia D., Ricciardi L., Melis M., et al. Parkinson's disease symptoms have a distinct impact on Caregivers' and Patients' stress: A study assessing the consequences of the COVID-19 lockdown. Movement Disorders Clinical Practice. 2020;7(7):865–867. 10.1002/mdc3.13030. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Paik S.H., Ahn J.S., Min S., Park K.C., Kim M.H. Impact of psychotic symptoms on clinical outcomes in delirium. PLoS One. 2018;13(7) 10.1371/journal.pone.0200538. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Palermo G., Tommasini L., Baldacci F., Del Prete E., Siciliano G., Ceravolo R. Impact of coronavirus disease 2019 pandemic on cognition in Parkinson's disease. Movement Disorders. 2020;35(10):1717–1718. 10.1002/mds.28254. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Pantell M., Rehkopf D., Jutte D., Syme S.L., Balmes J., Adler N. Social isolation: A predictor of mortality comparable to traditional clinical risk factors. American Journal of Public Health. 2013;103(11):2056–2062. 10.2105/ajph.2013.301261. [Abstract] [CrossRef] [Google Scholar]
- Parra A., Juanes A., Losada C.P., Álvarez-Sesmero S., Santana V.D., Martí I., et al. Psychotic symptoms in COVID-19 patients. A retrospective descriptive study. Psychiatry Research. 2020;291 10.1016/j.psychres.2020.113254. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Patel P.H., Hashmi M.F. StatPearls. StatPearls Publishing; Treasure Island (FL): 2021. (Copyright © 2021, StatPearls Publishing LLC) [Google Scholar]
- Picillo M., Palladino R., Erro R., Alfano R., Colosimo C., Marconi R., et al. The PRIAMO study: Age- and sex-related relationship between prodromal constipation and disease phenotype in early Parkinson's disease. Journal of Neurology. 2021;268(2):448–454. 10.1007/s00415-020-10156-3. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Poewe W., Seppi K., Tanner C.M., Halliday G.M., Brundin P., Volkmann J., et al. Parkinson disease. Nature Reviews. Disease Primers. 2017;3(1):17013. 10.1038/nrdp.2017.13. [Abstract] [CrossRef] [Google Scholar]
- Potvin O., Hudon C., Dion M., Grenier S., Préville M. Anxiety disorders, depressive episodes and cognitive impairment no dementia in community-dwelling older men and women. International Journal of Geriatric Psychiatry. 2011;26(10):1080–1088. 10.1002/gps.2647. [Abstract] [CrossRef] [Google Scholar]
- Prasad S., Holla V.V., Neeraja K., Surisetti B.K., Kamble N., Yadav R., et al. Parkinson's disease and COVID-19: Perceptions and implications in patients and caregivers. Movement Disorders. 2020;35(6):912–914. 10.1002/mds.28088. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Pun B.T., Badenes R., Heras La Calle G., Orun O.M., Chen W., Raman R., et al. Prevalence and risk factors for delirium in critically ill patients with COVID-19 (COVID-D): A multicentre cohort study. The Lancet. Respiratory Medicine. 2021;9(3):239–250. 10.1016/S2213-2600(20)30552-X. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Rangon C.-M., Krantic S., Moyse E., Fougère B. The vagal autonomic pathway of COVID-19 at the crossroad of Alzheimer's disease and aging: A review of knowledge. Journal of Alzheimer's Disease Reports. 2020;4(1):537–551. 10.3233/ADR-200273. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Robbins T.W., Cools R. Cognitive deficits in Parkinson's disease: A cognitive neuroscience perspective. Movement Disorders. 2014;29(5):597–607. 10.1002/mds.25853. [Abstract] [CrossRef] [Google Scholar]
- Rock P.L., Roiser J.P., Riedel W.J., Blackwell A.D. Cognitive impairment in depression: A systematic review and meta-analysis. Psychological Medicine. 2014;44(10):2029–2040. 10.1017/s0033291713002535. [Abstract] [CrossRef] [Google Scholar]
- Romano S., Savva G.M., Bedarf J.R., Charles I.G., Hildebrand F., Narbad A. Meta-analysis of the Parkinson's disease gut microbiome suggests alterations linked to intestinal inflammation. Npj. Parkinson's Disease. 2021;7(1):27. 10.1038/s41531-021-00156-z. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Rukavina K., Leta V., Sportelli C., Buhidma Y., Duty S., Malcangio M., et al. Pain in Parkinson's disease: New concepts in pathogenesis and treatment. Current Opinion in Neurology. 2019;32(4):579–588. 10.1097/wco.0000000000000711. [Abstract] [CrossRef] [Google Scholar]
- Salari M., Zali A., Ashrafi F., Etemadifar M., Sharma S., Hajizadeh N., et al. Incidence of anxiety in Parkinson's disease during the coronavirus disease (COVID-19) pandemic. Movement Disorders. 2020;35(7):1095–1096. 10.1002/mds.28116. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Saldanha D., Menon P., Chaudari B., Bhattacharya L., Guliani S. Acute psychosis: A neuropsychiatric dilemma. Industrial Psychiatry Journal. 2013;22(2):157–158. 10.4103/0972-6748.132933. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Santos-García D., Oreiro M., Pérez P., Fanjul G., Paz González J.M., Feal Painceiras M.J., et al. Impact of coronavirus disease 2019 pandemic on Parkinson's disease: A cross-sectional survey of 568 Spanish patients. Movement Disorders. 2020;35(10):1712–1716. 10.1002/mds.28261. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Schapira A.H.V., Chaudhuri K.R., Jenner P. Non-motor features of Parkinson disease. Nature Reviews. Neuroscience. 2017;18(8):509. 10.1038/nrn.2017.91. [Abstract] [CrossRef] [Google Scholar]
- Schirinzi T., Cerroni R., Di Lazzaro G., Liguori C., Scalise S., Bovenzi R., et al. Self-reported needs of patients with Parkinson's disease during COVID-19 emergency in Italy. Neurological Sciences. 2020;41(6):1373–1375. 10.1007/s10072-020-04442-1. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Shalash A., Roushdy T., Essam M., Fathy M., Dawood N.L., Abushady E.M., et al. Mental health, physical activity, and quality of life in Parkinson's disease during COVID-19 pandemic. Movement Disorders. 2020;35(7):1097–1099. 10.1002/mds.28134. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Silva Andrade B., Siqueira S., de Assis Soares W.R., de Souza Rangel F., Santos N.O., Dos Santos Freitas A., et al. Long-COVID and post-COVID health complications: An up-to-date review on clinical conditions and their possible molecular mechanisms. Viruses. 2021;13(4):700. 10.3390/v13040700. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Silva F., Brito B.B., Santos M.L.C., Marques H.S., Silva Júnior R.T.D., Carvalho L.S., et al. COVID-19 gastrointestinal manifestations: A systematic review. Revista da Sociedade Brasileira de Medicina Tropical. 2020;53 10.1590/0037-8682-0714-2020. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Silva A.R., Regueira P., Cardoso A.L., Baldeiras I., Santana I., Cerejeira J. Cognitive trajectories following acute infection in older patients with and without cognitive impairment: An 1-year follow-up study. Frontiers in Psychiatry. 2021;12 10.3389/fpsyt.2021.754489. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Simonet C., Tolosa E., Camara A., Valldeoriola F. Emergencies and critical issues in Parkinson's disease. Practical Neurology. 2020;20(1):15. 10.1136/practneurol-2018-002075. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Smith C.M., Gilbert E.B., Riordan P.A., Helmke N., von Isenburg M., Kincaid B.R., et al. COVID-19-associated psychosis: A systematic review of case reports. General Hospital Psychiatry. 2021;73:84–100. 10.1016/j.genhosppsych.2021.10.003. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Song J., Ahn J.H., Choi I., Mun J.K., Cho J.W., Youn J. The changes of exercise pattern and clinical symptoms in patients with Parkinson's disease in the era of COVID-19 pandemic. Parkinsonism & Related Disorders. 2020;80:148–151. 10.1016/j.parkreldis.2020.09.034. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Subramanian I., Farahnik J., Mischley L.K. Synergy of pandemics-social isolation is associated with worsened Parkinson severity and quality of life. NPJ Parkinsons Disease. 2020;6:28. 10.1038/s41531-020-00128-9. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Sulzer D., Antonini A., Leta V., Nordvig A., Smeyne R.J., Goldman J.E., et al. COVID-19 and possible links with Parkinson's disease and parkinsonism: From bench to bedside. NPJ Parkinsons Disease. 2020;6:18. 10.1038/s41531-020-00123-0. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Suzuki K., Numao A., Komagamine T., Haruyama Y., Kawasaki A., Funakoshi K., et al. Impact of the COVID-19 pandemic on the quality of life of patients with Parkinson's disease and their caregivers: A single-center survey in Tochigi prefecture. Journal of Parkinson's Disease. 2021;11(3):1047–1056. 10.3233/JPD-212560. [Abstract] [CrossRef] [Google Scholar]
- Tan A.H., Mahadeva S., Thalha A.M., Gibson P.R., Kiew C.K., Yeat C.M., et al. Small intestinal bacterial overgrowth in Parkinson's disease. Parkinsonism & Related Disorders. 2014;20(5):535–540. 10.1016/j.parkreldis.2014.02.019. [Abstract] [CrossRef] [Google Scholar]
- Taylor D.J., Lichstein K.L., Durrence H.H., Reidel B.W., Bush A.J. Epidemiology of insomnia, depression, and anxiety. Sleep. 2005;28(11):1457–1464. 10.1093/sleep/28.11.1457. [Abstract] [CrossRef] [Google Scholar]
- Ticinesi A., Cerundolo N., Parise A., Nouvenne A., Prati B., Guerra A., et al. Delirium in COVID-19: Epidemiology and clinical correlations in a large group of patients admitted to an academic hospital. Aging Clinical and Experimental Research. 2020;32(10):2159–2166. 10.1007/s40520-020-01699-6. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Tuzun S., Keles A., Okutan D., Yildiran T., Palamar D. Assessment of musculoskeletal pain, fatigue and grip strength in hospitalized patients with COVID-19. European Journal of Physical and Rehabilitation Medicine. 2021;57(4):653–662. 10.23736/s1973-9087.20.06563-6. [Abstract] [CrossRef] [Google Scholar]
- van der Heide A., Meinders M.J., Bloem B.R., Helmich R.C. The impact of the COVID-19 pandemic on psychological distress, physical activity, and symptom severity in Parkinson's disease. Journal of Parkinson's Disease. 2020;10(4):1355–1364. 10.3233/jpd-202251. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- van Wamelen D.J., Wan Y.M., Ray Chaudhuri K., Jenner P. Stress and cortisol in Parkinson's disease. International Review of Neurobiology. 2020;152:131–156. 10.1016/bs.irn.2020.01.005. [Abstract] [CrossRef] [Google Scholar]
- Varatharaj A., Thomas N., Ellul M.A., Davies N.W.S., Pollak T.A., Tenorio E.L., et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: A UK-wide surveillance study. The Lancet. Psychiatry. 2020;7(10):875–882. 10.1016/S2215-0366(20)30287-X. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Vardy E.R., Teodorczuk A., Yarnall A.J. Review of delirium in patients with Parkinson's disease. Journal of Neurology. 2015;262(11):2401–2410. 10.1007/s00415-015-7760-1. [Abstract] [CrossRef] [Google Scholar]
- Villapol S. Gastrointestinal symptoms associated with COVID-19: Impact on the gut microbiome. Translational Research. 2020;226:57–69. 10.1016/j.trsl.2020.08.004. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Wakefield B.J. Risk for acute confusion on hospital admission. Clinical Nursing Research. 2002;11(2):153–172. 10.1177/105477380201100205. [Abstract] [CrossRef] [Google Scholar]
- Wan D., Du T., Hong W., Chen L., Que H., Lu S., et al. Neurological complications and infection mechanism of SARS-COV-2. Signal Transduction and Targeted Therapy. 2021;6(1):406. 10.1038/s41392-021-00818-7. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Webster R., Holroyd S. Prevalence of psychotic symptoms in delirium. Psychosomatics. 2000;41(6):519–522. 10.1176/appi.psy.41.6.519. [Abstract] [CrossRef] [Google Scholar]
- Weems C.F., Costa N.M., Dehon C., Berman S.L. Paul Tillich's theory of existential anxiety: A preliminary conceptual and empirical examination. Anxiety, Stress, and Coping. 2004;17(4):383–399. 10.1080/10615800412331318616. [CrossRef] [Google Scholar]
- Weng L.M., Su X., Wang X.Q. Pain symptoms in patients with coronavirus disease (COVID-19): A literature review. Journal of Pain Research. 2021;14:147–159. 10.2147/jpr.S269206. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Whitworth S.R., Loftus A.M., Skinner T.C., Gasson N., Barker R.A., Bucks R.S., et al. Personality affects aspects of health-related quality of life in Parkinson's disease via psychological coping strategies. Journal of Parkinson's Disease. 2013;3(1):45–53. 10.3233/JPD-120149. [Abstract] [CrossRef] [Google Scholar]
- Woo M.S., Malsy J., Pöttgen J., Seddiq Zai S., Ufer F., Hadjilaou A., et al. Frequent neurocognitive deficits after recovery from mild COVID-19. Brain Communications. 2020;2(2):fcaa205. 10.1093/braincomms/fcaa205. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- World Health Organization . Retrieved February 4, 2022, from; 2022. https://covid19.who.int/region/searo/country/in [Google Scholar]
- Xia Y., Kou L., Zhang G., Han C., Hu J., Wan F., et al. Investigation on sleep and mental health of patients with Parkinson's disease during the coronavirus disease 2019 pandemic. Sleep Medicine. 2020;75:428–433. 10.1016/j.sleep.2020.09.011. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Xiao Q., Chen S., Le W. Hyposmia: A possible biomarker of Parkinson's disease. Neuroscience Bulletin. 2014;30(1):134–140. 10.1007/s12264-013-1390-3. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Xu Y., Surface M., Chan A.K., Halpern J., Vanegas-Arroyave N., Ford B., et al. COVID-19 manifestations in people with Parkinson's disease: A USA cohort. Journal of Neurology. 2021;1-7 10.1007/s00415-021-10784-3. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Yan A., Bryant E.E. StatPearls. StatPearls Publishing; Treasure Island (FL): 2021. (Copyright © 2021, StatPearls Publishing LLC) [Google Scholar]
- Ye Q., Wang B., Zhang T., Xu J., Shang S. The mechanism and treatment of gastrointestinal symptoms in patients with COVID-19. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2020;319(2):G245–G252. 10.1152/ajpgi.00148.2020. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Yusuf F., Fahriani M., Mamada S.S., Frediansyah A., Abubakar A., Maghfirah D., et al. Global prevalence of prolonged gastrointestinal symptoms in COVID-19 survivors and potential pathogenesis: A systematic review and meta-analysis. F1000Res. 2021;10:301. 10.12688/f1000research.52216.1. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Zhou H., Lu S., Chen J., Wei N., Wang D., Lyu H., et al. The landscape of cognitive function in recovered COVID-19 patients. Journal of Psychiatric Research. 2020;129:98–102. 10.1016/j.jpsychires.2020.06.022. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Zipprich H.M., Teschner U., Witte O.W., Schonenberg A., Prell T. Knowledge, attitudes, practices, and burden during the COVID-19 pandemic in people with Parkinson's disease in Germany. Journal of Clinical Medicine. 2020;9(6) 10.3390/jcm9061643. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1016/bs.irn.2022.04.001
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9270874
Citations & impact
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/133069820
Article citations
Circadian re-set repairs long-COVID in a prodromal Parkinson's parallel: a case series.
J Med Case Rep, 18(1):496, 23 Oct 2024
Cited by: 0 articles | PMID: 39438926 | PMCID: PMC11520186
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Covid-19 and Parkinson's disease: Acute clinical implications, long-COVID and post-COVID-19 parkinsonism.
Int Rev Neurobiol, 165:63-89, 08 Aug 2022
Cited by: 15 articles | PMID: 36208907 | PMCID: PMC9357514
The impact of COVID-19 on palliative care for people with Parkinson's and response to future pandemics.
Expert Rev Neurother, 21(6):615-623, 19 May 2021
Cited by: 9 articles | PMID: 33905283
Parkinson's disease and Covid-19: Is there an impact of ethnicity and the need for palliative care.
Int Rev Neurobiol, 165:229-249, 27 Apr 2022
Cited by: 2 articles | PMID: 36208902 | PMCID: PMC9042419
Post-COVID-19 Depressive Symptoms: Epidemiology, Pathophysiology, and Pharmacological Treatment.
CNS Drugs, 36(7):681-702, 21 Jun 2022
Cited by: 62 articles | PMID: 35727534 | PMCID: PMC9210800
Review Free full text in Europe PMC