Abstract
Free full text

Studies on the Selectivity Between Nickel-Catalyzed 1,2-Cis-2-Amino Glycosylation of Hydroxyl Groups of Thioglycoside Acceptors with C(2)-Substituted Benzylidene N-Phenyl Trifluoroacetimidates and Intermolecular Aglycon Transfer of the Sulfide Group
Abstract
The stereoselective synthesis of saccharide thioglycosides containing 1,2-cis-2-amino glycosidic linkages is challenging. In addition to the difficulties associated with achieving high α-selectivity in the formation of 1,2-cis-2-amino glycosidic bonds, the glycosylation reaction is hampered by undesired transfer of the anomeric sulfide group from the glycosyl acceptor to the glycosyl donor. Overcoming these obstacles will pave the way for the preparation of oligosaccharides and glycoconjugates bearing the 1,2-cis-2-amino glycosidic linkages because the saccharide thioglycosides obtained can serve as donors for another coupling iteration. This approach streamlines selective deprotection and anomeric derivatization steps prior to the subsequent coupling event. We have developed an efficient approach for the synthesis of highly yielding and α-selective saccharide thioglycosides containing 1,2-cis-2-amino glycosidic bonds, via cationic nickel-catalyzed glycosylation of thioglycoside acceptors bearing the 2-trifluoromethylphenyl aglycon with N-phenyl trifluoroacetimidate donors. The 2-trifluoromethylphenyl group effectively blocks transfer of the anomeric sulfide group from the glycosyl acceptor to the C(2)-benzylidene donor and can be easily installed and activated. The current method also highlights the efficacy of the nickel catalyst selectively activating the C(2)-benzylidene imidate group in the presence of the anomeric sulfide group on the glycosyl acceptors.
INTRODUCTION
Alkyl and aryl thioglycosides have been widely utilized as both the acceptors and donors in the multi-step synthesis of oligosaccharides and glycoconjugates.1 They are versatile in that their anomeric sulfide groups are easy to install and stable under many reaction conditions.2 As a result, the sulfide groups not only serve as efficient protecting groups of anomeric centers but also act as glycosyl acceptors in combination with a variety of glycosyl donors such as trichloroacetimidates, halides and sulfoxides.3 The resulting saccharide thioglycosides can be subsequently activated for another coupling iteration to generate the corresponding oligosaccharides or glycoconjugates.3,4 Despite the widespread synthetic utility of thioglycosides, intermolecular aglycon transfer can be a serious side reaction. When an unreactive thioglycoside acceptor is employed in the coupling process, the anomeric sulfide group can be transferred from the glycosyl acceptor to the glycosyl donor to give another thioglycoside.6 To prevent aglycon transfer, Li and Gildersleeve have recently reported the use of 2,6-dimethylphenyl (DMP) group as an efficient aglycon for thioglycoside acceptors.5 This bulky group effectively blocks intermolecular aglycon transfer6 of the anomeric sulfur atom in the glycosylation reaction.
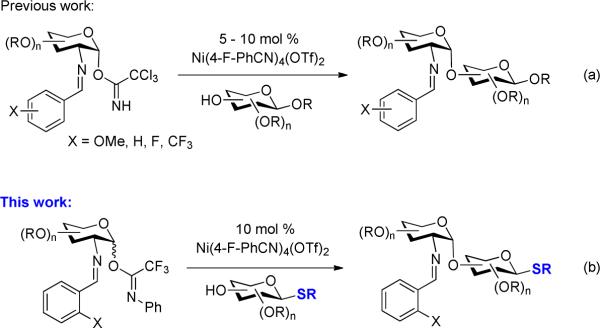
We recently developed a novel strategy for the synthesis of 1,2-cis-2-aminosugars via nickel-catalyzed coupling with the C(2)-N-substituted benzylidene trichloroacetimidates (Scheme 1a).7,8 This method utilizes the nature of the nickel-ligand complex and metal-bound functional group at the C(2)-position of glycosyl donors to control the α-selectivity and provide high yielding oligosaccharides and glycoconjugates from a wide variety of glycosyl donors and acceptors.
In an effort to expand the capabilities of this method, we targeted the development of a variant proceeding via chemoselective coupling to the hydroxyl groups of thioglycosides, as depicted in Scheme 1b. A number of challenges have to be addressed in developing such a chemoselective glycosylation process. For instance, the nickel catalyst could fail to promote the coupling due to the displacement of metal-bound benzonitrile ligand by the anomeric sulfide group, producing an inactive nickel catalyst.9 Second, the trihaloacetimidate group could undergo [1,3]-rearrangement to provide the corresponding glycosyl trihaloacetamide.10 Third, intermolecular aglycon transfer of the anomeric sulfide group from the glycosyl acceptor to the glycosyl donor provides undesired thioglycoside.6 We report herein an efficient approach for the preparation of saccharides containing 1,2-cis-2-amino glycosidic linkages via nickel-catalyzed α-chemoselective glycosylation of the hydroxyl groups of thioglycoside acceptors with C(2)-benzylidene N-phenyl trifluoroacetimidates (Scheme 1b). This strategy overcomes the challenges associated with intermolecular aglycon transfer of the anomeric sulfide group from the acceptors to the C(2)-benzylidene imidate donors and retains the unique features of the nickel catalyst to control the α-selectivity at the newly-formed glycosidic bond.
RESULTS AND DISCUSSION
Optimization Studies
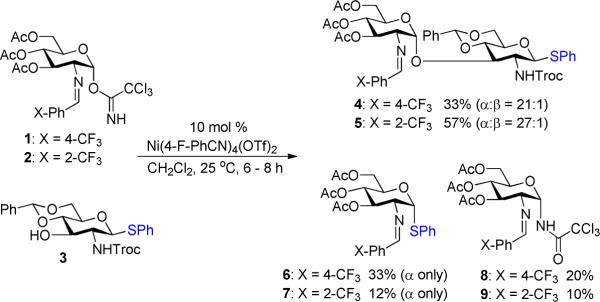
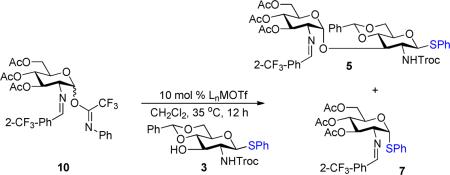
We initially explored the nickel-catalyzed glycosylation of N-Troc protected thioglycoside 35f,g with the C(2)-N-p-trifluoromethyl-benzylidene trichloroacetimidate 1 (Scheme 1) to probe issues of both aglycon transfer and selectivity. Under previous nickel conditions (10 mol% Ni(4-F-PhCN)4(OTf)2 at 25 °C in CH2Cl2),7 disaccharide 4 (Scheme 2) was isolated in 40% along with aglycon transfer product 6 (26%) and [1,3]-rearrangement trichloroacetamide 8 (20%). Switching to C(2)-N-o-trifluoromethyl-benzylidene donor 2 improved both the yield and α-selectivity of disaccharide 5 (57%) and decreased the amount of transfer product 11 (12%) and trichloroacetamide 9 (10%). Formation of the rearrangement products 8 and 9 indicate the disadvantage of utilizing trichloroacetimidates as glycosyl donors.
To prevent [1,3]-rearrangement of the imidate group, the N-phenyl trifluoroacetimidate donor 10 (Table 1) was chosen due to its attenuated reactivity in comparison to trichloroacetimidates 1 and 2 (Scheme 2).11 Accordingly, coupling of thioglycoside 3 (Table 1) with 10 was first examined at 25 °C, and the reaction was sluggish. Warming the reaction to 35 °C accelerated the coupling process and provided 5 (entry 1) in 58% yield as a single α-isomer. The undesired rearrangement acetamide (e.g. 9) was not observed. In this experiment, both α- and β-isomers of donor 10 reacted, highlighting another advantage of using this glycosyl donor.12 A significant amount of aglycon transfer product 7 (14%, entry 1), however, was still obtained in the reaction.
Table 1
Studies with N-Phenyl Trifluoroacetimidatea
| entry | acceptors | donor/acceptor ratio | disaccharides yield (%)b (α:β)c | thioglycosides yield(%) |
|---|---|---|---|---|
| 1 | 3: R = Ph | 1/2 | 5:58 (α only) | 7: 14 |
| 2 | 11: R = Et | 1/2 | 18: 43(10:1) | 25: 22 |
| 3 | 12: R = 1-Naphthyl | 1/2 | 19: 59 (α only) | 26: 10 |
| 4 | 13: R = 4-Me-Ph | 1/2 | 20: 38 (11:1) | 27:20 |
| 5 | 14: R = 4-MeO-Ph | 1/2 | 21: 37 (11:1) | 28: 30 |
| 6 | 15: R = 4-F-Ph | 1/2 | 22: 58 (20:1) | 29: 9 |
| 7 | 16: R = 2-F-Ph | 1/2 | 23: 60 (α only) | 30: 4 |
| 8 | 17: R = 2-CF3-Ph | 1/2 | 24:61 (α only) | 31: 0 |
| 9 | 17: R = 2-CF3-Ph | 2/1 | 24: 58 (α only) | 31: 0 |
Thus, the key to the optimization is to minimize aglycon transfer. Unfortunately, we were unable to install the DMP group5 onto the anomeric center of N-Troc glucosamine. We then systematically examined the steric and electronic nature of the substituents on the phenyl ring of a number of thioglycosides 12–17 (Table 1) in the coupling with imidate 10. While the electron-rich 4-methoxyphenyl aglycon 14 (entry 5) significantly increased the amount of transfer product 28, the electron-poor groups 15–17 (entries 6–8) nearly blocked transfer. The most efficient acceptor was 2-trifluoromethylphenyl thioglycoside 17 (entry 8), which completely prevented transfer and provided disaccharide 24 (entry 8) in 61% yield and exclusively as the α-isomer. Even with two equivalents of donor 10, no aglycon transfer product 31 (entry 9) was observed in the reaction. Overall, the 2-trifluoromethylphenyl group is an excellent alternative aglycon for thioglycoside acceptor. In addition, the nickel catalyst not only selectively activates the C(2)-benzylidene imidate 10 in the presence of the anomeric sulfide group on the glycosyl acceptor 17 but also effectively controls the α-anomeric selectivity in the synthesis of disaccharide thioglycoside 24 containing 1,2-cis-2-amino glycosidic bond.
To evaluate the unique features of the nickel catalyst as the effective activating agent to promote the glycosylation reaction, a number of catalysts (Table 2) were screened in the coupling of 2-trifluoromethylphenyl thioglycoside 3 with imidate donor 10. Using TMSOTf (entry 1) resulted in 13% of disaccharide 5 with α:β = 10:1 and 40% of transfer product 7. Similar results were obtained with a milder Lewis acid, Cu(OTf)2 (entry 2). Use of both Rh(COD)2OTf and Ir(COD)2OTf catalysts (entries 3 and 4) provided 5 in lower yield (43 – 45%) and α-selectivity (α:β = 6:1 – 8:1) in comparison to the nickel catalyst whereas 7 was obtained in 58% yield and as a single α-isomer (Table 1, entry 1). In addition, employing iridium as the activating agent significantly increased the amount of transfer product 7 (14% → 26%). The palladium catalyst (entry 5), Pd(4-F-PhCN)2OTf2, decreased the yield of disaccharide 5 (40%) but increased the yield of transfer product 7 (14%→24%). Gold catalyst, Ph3PAuOTf (entry 6), provided exclusive α-disaccharide 5 in 43% yield, but a significant amount of sulfide transfer 7 (24%) was still obtained in the reaction. Although less than 1% of transfer product 7 was observed in the reaction using Fe(OTf)2 as the catalyst (entry 7), the desired disaccharide 5 was only isolated in 11% yield.
Table 2
Activation of Glycosyl Imidate with Various Catalysts.a
| Entry | Catalysts | Disaccharide 5 Yield (%)b (α:β)c | Thioglyooside 7 Yield (%) |
|---|---|---|---|
| 1 | TMSOTf | 13 (10:1) | 40 |
| 2 | Cu(OTf)2 | 26 (10:1) | 30 |
| 3 | Rh(COD)2OTf | 43(6:1) | 20 |
| 4 | lr(COD)2OTf | 45 (8:1) | 26 |
| 5 | Pd(4-F-PhCN)2(OTf) | 36(20:1) | 23 |
| 6 | Ph3PAuOTf | 43 (α only) | 24 |
| 7 | Fe(OTf)2 | 11 (11:1) | 1 |
Substrate Scope
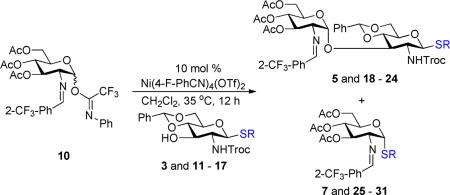
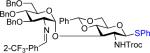
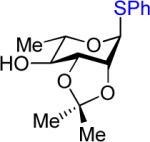
We next examined the scope of the glycosylation of a variety of thioglycosides with armed and disarmed imidates 10, 32, and 33 (Table 3). In contrast to disarmed donor 10 (Table 1, entry 1), coupling of armed donor 33 (Table 3, entry 1) with phenyl thioglycoside 3 resulted in no transfer product 47; disaccharide 39 (entry 1) was isolated in 70% yield, albeit in lower α-selectivity (α:β = 6:1). Similarly, coupling of phenyl d-galactosyl thioglycoside 34 with both donors 10 and 32 (entries 2 and 3) provided a large quantity of sulfide transfer products 7 and 49, respectively. The armed donor 32 provided less aglycon transfer than the disarmed donor 10 (9% vs. 24%). The 2-trifluoromethylphenyl aglycon was then incorporated into d-galactosyl thioglycoside 35 (entry 4).6 No trace amount of transfer product 31 (entry 4) was observed, and exclusive α-disaccharide 42 was isolated in 71% yield. The efficacy of the nickel method was further evaluated with l-rhamnose acceptors 36 and 37 (entries 5 and 8). As anticipated, although glycosylation of the phenyl thioglycoside acceptor 36 with both disarmed donor 10 (entry 5) and armed donor 32 (entry 6) provided disaccharides 43 and 44, respectively, in good yields (72 – 81%) and with complete α-selectivity, a significant amount of transfer products 7 (15%) and 49 (9%) was still observed in the coupling process. On the other hand, the electron deficient 2-trifluoromethylphenyl thioglycoside 37 completely blocked sulfide transfer (entries 7 and 8), providing disaccharides 45 and 46 in high yield (78 – 98%) and as single α-isomers. To further compare, the DMP group was incorporated into l-rhamnose thioglycoside 38 (entry 9). In agreement with previous studies,5 no transfer product 51 was obtained in the reaction. The modified disaccharide 47 (entry 9), however, was obtained in lower yield than disaccharide 45 (entry 5) bearing the 2-trifluoromethylphenyl aglycon. Overall, the results clearly illustrate that the electron-withdrawing 2-trifluoromethylphenyl aglycon completely prevents transfer of the anomeric sulfide group from thioglycoside acceptors to both disarmed and armed N-phenyl trifluoroacetimidate donors.
Table 3
Coupling of Thioglycosides with Glucosamine Donorsa

| entry | donors | acceptors | disaccharides yieldd(α:β)e | thioglycosides (yield)f |
|---|---|---|---|---|
 | ||||
| 1 | 33 | 3 | 39:64%(6:1)b | 48:0% |
 |
 | |||
| 2 | 10 | 34 | 40: R = Ac 55% (α only)b | 7: 24% |
| 3 | 32 | 34 | 41:R=Bn 61% (α only)b | 49: 8% |
 |
 | |||
| 4 | 10 | 35 | 42:71%(α only)c | 31: 0% |
 |
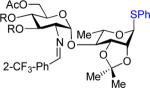 | |||
| 5 | 10 | 36 | 43: R = Ac 81% (α only)b | 7:15% |
| 6 | 32 | 36 | 44: R = Bn 72% (α only)b | 49: 9% |
 |
 | |||
| 7 | 10 | 37 | 45: R = Ac 98% (α onlyb | 31: 0% |
| 8 | 32 | 37 | 46: R = Bn 78% (α only)b | 50: 0% |
 |
 | |||
| 9 | 10 | 38 | 47: 74% (α only)b | 51:0% |
The utility of the current method was further explored with galactosamine donors 52 and 53 (Table 4). Coupling of phenyl thioglycoside 3 with both disarmed and armed galactosamine donors 52 and 53 (entries 1 and 2) provided disaccharides 54 and 55, respectively, in moderate yield (56 – 67%) and with excellent α-selectivity (α:β = 14:1 – >20:1). As expected, a significant amount of transfer products 60 and 61 (6 – 16%) was also isolated in the reaction. On the other hand, employing 2-trifluoromethylphenyl thioglycoside 17 (entry 3) as a glycosyl acceptor completely blocked formation of transfer product 62 and provided α-disaccharide 56 (entry 3) in 73% yield. Derivative of disaccharides 5456 (entries 1–3), GalNAc-α-(1→3)-GlcNAc, is a part of the O-polysaccharide present in the outer membrane of Gram-negative bacteria such as Salmonella enterica13 and Providencia rustigianii.14S. enterica is a major pathogen of humans and animals.11P. rustigianii invades human intestinal mucosa and causes stomach infection particularly in children.12 Next, phenyl thioglycoside 34 (entries 4 and 5) was investigated with both glycosyl donors 52 and 53, and a significant amount of transfer products 60 and 61 were obtained in the glycosylation reaction. Under nickel conditions, the higher amount of transfer product 61 (40%, entry 5) was obtained with partially armed donor 53 than that of transfer product 60 (24%, entry 4) with disarmed donor 52. Thioglycoside 35 (entry 6) bearing the 2-trifluoromethylphenyl aglycon was then explored with this problematic reaction. As expected, the modified disaccharide 59 (entry 6) was isolated in 67% yield and with exclusively α-selectivity. Again, transfer product 62 was not observed with this 2-trifluoromethylphenyl acceptor 35. Disaccharides 57–59 (entries 4–5), derivatives of GalNAc-α-(1→3)-Gal, are vital disaccharide units of human blood group A antigens.15
Table 4
Coupling of Thioglycosides with Galacto samine Donorsa
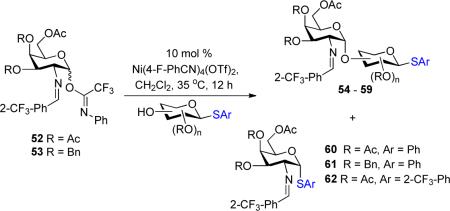
| entry | donors | acceptors | disaccharides yieldd (α:β)e | thioglycosides (yield)f |
|---|---|---|---|---|
 | ||||
| 1 | 52 | 3 | 54: R = Ac 67% (α only)b | 60: 16% |
| 2 | 53 | 3 | 55: R = Bn 56% (14:1)b | 61: 6% |
 | ||||
| 3 | 52 | 17 | 56: 73% (α only)b | 62: 0% |
 | ||||
| 4 | 52 | 34 | 57: R = Ac 52% (8:1)b | 60: 24% |
| 5 | 53 | 34 | 58: R=Bn 51% (α only)b | 61:40% |
 | ||||
| 6 | 52 | 35 | 59: 62% (α only)b | 62: 0% |
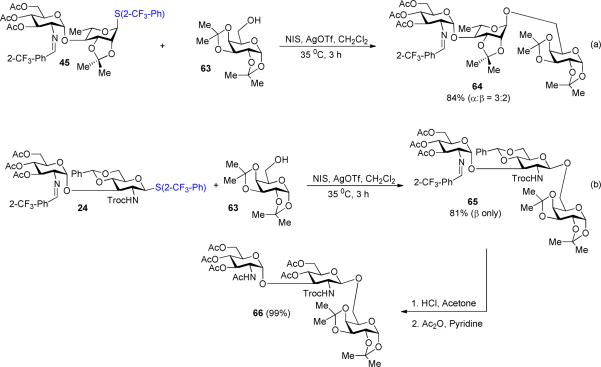
One of the key concerns in reducing the reactivity of the sulfide aglycon to prevent intermolecular transfer is that the modified arylthiol group cannot be efficiently activated. To illustrate that the 2-trifluoromethylphenyl aglycon can be sufficiently activated with thiophilic reagents (Scheme 3), disaccharide thioglycoside donor 45 was treated with the reagent combination of NIS and AgOTf.16,17 Subsequent addition of a galactosyl acceptor 63 provided trisaccharide 64 in 84% yield, validating the ability of the 2-trifluoromethylphenyl group as an excellent alternative aglycon for thioglycosides. Encouraged by this result, we further explored the glycosylation of glycosyl acceptor 63 with less reactive disaccharide thioglycoside donor 24 (Scheme 3b) under similar nickel conditions. Gratifyingly, the coupling process proceeded smoothly to provide the desired trisaccharide 65 in 81% yield and with exclusive β-selectivity. The results obtained in Scheme 3 also illustrate the ability of the C(2)-N-substituted benzylidene functionality to act as the efficient protecting group. Next, it is essential to determine that these benzylidene groups could be converted to the corresponding N-acetyl or other functional groups. Accordingly, treatment of 65 with HCl, acetone/CH2Cl2 at 25°C for less than 5 min provided the corresponding amine salt along with the concomitant removal of the benzylidene protecting group. To ease the purification process, the resulting amine salt intermediate was acetylated to afford the fully protected trisaccharide 66 (Scheme 3b) in 99% yield over two steps.
Currently, the most common method for the synthesis of 1,2-cis-2-aminosugars employs glycosyl donors bearing the C(2)-azido functionality.18 To compare the ability of the nickel catalyst to efficiently activate the C(2)-azido donor, a series of glycosylations were performed with glycosyl N-phenyl trifluoroacetimidate 67 (Scheme 4).9,19 Accordingly, coupling of phenyl thioglycoside acceptor 3 with imidate 67 in the presence of 10 mol% Ni(4-F-PhCN)4(OTf)2 provided sulfide transfer product 69 (40%) as the major product (Scheme 4a). The desired disaccharide 68 was obtained in 18% yield, albeit with excellent α-selectivity. Although transfer product was not detected in the reaction with the 2-trifluoromethyl-phenyl thioglycoside acceptor 17, only 13% of the desired disaccharide 67 was isolated (Scheme 4b).20 When l-rhamnoside 36 was employed as the glycosyl donors in the reaction with C(2)-azido donor 67 (Scheme 4c), higher amount of transfer product 69 (28% vs. 15%) and lower yield of the desired disaccharide 71 (49% vs. 81%) were obtained than those with C(2)-benzylidene donor 10 (Table 3, entry 5). Overall, these results suggest that the nickel catalyst, Ni(4-F-PhCN)4(OTf)2, is the more efficient activating reagent for C(2)-N-substituted benzylideneamino donors than that for C(2)-azido donors.
CONCLUSION
In summary, we have developed an efficient strategy to chemoselective glycosylation for the preparation of various saccharide thioglycoside building blocks in moderate to good yields and with excellent levels of α-selectivity. This approach employs a method of nickel-catalyzed 1,2-cis-2-amino glycosylation of thioglycoside acceptors bearing the 2-trifluoromethylphenyl aglycon with armed and disarmed C(2)-substituted N-phenyl trifluoroacetimidate donors. The resulting saccharide thioglycosides serve as glycosyl donors for another coupling iteration to generate oligosaccharides. Overall, the 2-trifluoromethylphenyl functionality is a more efficient aglycon than its phenyl counterpart to completely prevent transfer of the sulfide group from the glycosyl acceptor to the C(2)-benzylidene N-phenyl trifluoroacetimidate donors. In addition, it can be easily introduced onto the glycosyl acceptor and subsequently activated. This approach to the stereoselective synthesis of saccharide thioglycosides also highlights the ability of the C(2)-N-substituted benzylidene functionality as the efficient protecting group. Efforts are underway in our group to adapt this strategy for the preparation of biologically active oligosaccharides and glycoconjugates.
EXPERIMENTAL SECTION
General Procedure for the Preparation of Glycosyl Donors: 3,4,6-Tri-O-Acetyl-2-Deoxy-2-o-Trifluoromethylbenzylideneamino-d-Glucopyranosyl N-Phenyl Trifluoroacetimidate 10
A 100 mL RBF was charged with triacetyl d-glucosamine hemiacetal 10A (4.9 g, 10.6 mmol, 1.0 equiv.),7b 2,2,2-trifluoro-N-phenyl-ethanimidoyl chloride (6.66 g, 31.9 mmol, 3.0 equiv.), K2CO3 (2.93 g, 21.2 mmol, 2.0 equiv.) and acetone (30.0 mL). The solution was stirred at room temperature overnight. When the reaction was complete as monitored by TLC (hexane/ethyl acetate = 3/1), the reaction mixture was filtered, evaporated, and purified by flash chromatography on silica gel (hexane/ethyl acetate = 5/1 → 3/1 with 1% Et3N) to afford 10 (5.26 g, 80%, α:β = 1:4) as a viscous oil. 10α:1H NMR (CDCl3, 400 MHz): δ = 8.68 (s, 1 H), 8.22 (d, J = 7.6 Hz, 1 H), 7.73–7.52 (m, 3 H), 7.25–7.13 (m, 2 H), 7.12–6.98 (m, 1 H), 6.88–6.65 (m, 2 H), 6.44 (brs, 1 H), 6.65 (t, J = 10.0 Hz, 1 H), 5.23 (app t, J = 10.0 Hz, 1 H), 4.48–4.27 (m, 2 H), 4.22–4.08 (m, 1 H), 3.88–3.77 (m, 1 H), 2.10 (s, 3 H), 2.05 (s, 3 H), 1.89 (s, 3 H); 13C NMR (CDCl3, 100 MHz): δ = 170.4, 169.7, 169.5, 161.5, 143.2, 133.1, 132.1, 131.0, 129.4 (q, JC-F = 30.5 Hz), 128.64, 128.59, 125.6 (q, JC-F = 5.6 Hz), 124.3, 124.0 (d, JC-F = 272 Hz), 119.4, 94.7, 71.1, 70.5, 68.0, 61.7, 20.55, 20.50, 20.2; IR (film, cm−1): ν = 2963, 1752, 1644, 1367, 1315, 1212; HRMS (ESI): calc. for C28H26F6N2O8Na (M+Na): 655.1491; found: 655.1497. 10β:1H NMR (CDCl3, 400 MHz): δ = 8.68 (d, J = 2.4 Hz, 1 H), 8.11 (d, J = 7.6 Hz, 1 H), 7.71 (d, J = 7.2 Hz, 1 H), 7.68–7.51 (m, 2 H), 7.38–7.27 (m, 2 H), 7.19–7.07 (m, 1 H), 6.82 (d, J = 7.6 Hz, 2 H), 6.02 (brs, 1 H), 5.46 (t, J = 10.0 Hz, 1 H), 5.19 (app t, J = 10.0 Hz, 1 H), 4.34 (dd, J = 12.4, 4.8 Hz, 1 H), 4.17 (dd, J = 12.4, 2.0 Hz, 1 H), 3.93–3.76 (m, 1 H), 3.71 (t, J = 8.8 Hz, 1 H), 2.10 (s, 3 H), 2.04 (s, 3 H), 1.93 (s, 3 H); 13C NMR (CDCl3, 100 MHz): δ = 170.4, 169.5, 143.2, 133.3, 132.1, 130.9, 129.3 (q, JC-F = 31.0 Hz), 128.7, 128.6, 125.6 (q, JC-F = 5.5 Hz), 124.4, 124.0 (d, JC-F = 272 Hz), 119.2, 95.7, 73.3, 72.9, 72.7, 68.0, 61.7, 20.53, 20.47, 20.2; IR (film, cm−1): ν = 2887, 1752, 1645, 1489, 1315, 1231; HRMS (ESI): calc. for C28H26F6N2O8Na (M+Na): 655.1491; found: 655.1511.
3,4,6-Tri-O-Acetyl-2-Deoxy-2-o-Trifluoromethylbenzylideneamino-d-Galactopyranosyl N-Phenyl Trifluoroacetimidate 52
Viscous oil: 276 mg, 94%, α:β = 1:4; 52α:1H NMR (CDCl3, 400 MHz): δ = 8.70 (s, 1 H), 8.22 (d, J = 7.6 Hz, 1 H), 7.70 (d, J = 7.6 Hz, 1 H), 7.65–7.52 (m, 2 H), 7.31–7.18 (m, 2 H), 7.10–7.02 (m, 1 H), 6.89–6.66 (m, 2 H), 6.50 (brs, 1 H), 5.68–5.56 (m, 1 H), 5.51 (dd, J = 10.4, 2.8 Hz, 1 H), 4.53 (app t, J = 6.0 Hz, 1 H), 4.34–4.11 (m, 2 H), 4.09–3.93 (m, 1 H), 2.20 (s, 3 H), 2.08 (s, 3 H), 1.90 (s, 3 H); 13C NMR (CDCl3, 100 MHz): δ = 170.3, 170.0, 169.7, 161.6, 143.3, 133.3 132.1, 130.9, 129.2 (q, JC-F = 31.0 Hz), 128.7, 128.5, 125.6 (q, JC-F = 5.5 Hz), 124.6, 124.1 (d, JC-F = 272 Hz), 119.4, 95.3, 69.4, 68.1, 66.3, 66.2, 61.6, 20.7, 20.6, 20.3; IR (film, cm−1): ν = 2968, 1750, 1713, 1645, 1488, 1314; HRMS (ESI): calc. for C28H26F6N2O8Na (M+Na): 655.1491; found: 655.1502. 52β:1H NMR (CDCl3, 400 MHz): δ = 8.71 (s, 1 H), 8.10 (d, J = 7.6 Hz, 1 H), 7.69 (d, J = 7.6 Hz, 1 H), 7.66–7.48 (m, 2 H), 7.37–7.20 (m, 2 H), 7.17–7.02 (m, 1 H), 6.89–6.71 (m, 2 H), 6.00 (brs, 1 H), 5.53–5.41 (m, 1 H), 5.38–5.12 (m, 1 H), 4.42–4.16 (m, 2 H), 4.11–3.98 (m, 1 H), 3.84 (t, J = 8.4 Hz, 1 H), 2.20 (s, 3 H), 2.02 (s, 3 H), 1.91 (s, 3 H); 13C NMR (CDCl3, 100 MHz): δ = 170.2, 170.1, 169.6, 162.5, 143.2, 133.4, 132.1, 130.8, 129.2 (q, JC-F = 31.0 Hz), 128.7, 128.5, 125.6 (q, JC-F = 5.7 Hz), 124.4, 124.0 (d, JC-F = 272 Hz), 119.1, 96.0, 71.9, 70.9, 69.1, 65.5, 61.2, 20.6, 20.5, 20.2; IR (film, cm−1): ν = 2932, 1747, 1721, 1646, 1488, 1371, 1314, 1209; HRMS (ESI): calc. for C28H26F6N2O8Na (M+Na): 655.1491; found: 655.1501.
1,6-Di-O-tert-Butyldimethylsilyl-2-Deoxy-2-p-Methoxybenzylideneamino-d-Galactopyranoside 53B
A 250 mL oven-dried RBF was charged with 53A (3.36 g, 11.3 mmol, 1 equiv.),7b TBSCl (3.58 g, 23.7 mmol, 2.1 equiv.), imidazole (1.69 g, 24.9 mmol, 2.2 equiv.) and THF/DMF (40 mL/10 mL). The solution was stirred at room temperature overnight. The solution was diluted with CH2Cl2/MeOH (20:1), washed with brine (2 × 50 mL). The aqueous phase was then extracted with CH2Cl2/MeOH (20:1, 2 × 100 mL). The organic phase was combined, concentrated in vacuo. The resulting residue was purified by silica gel flash chromatography (hexane/ethyl acetate = 10/1 → 5/1 → 1/1 with 1% Et3N) to afford 53B (1.94 g, 35%) as a viscous oil. 1H NMR (CDCl3, 400 MHz): δ = 8.26 (s, 1H), 7.67 (d, J = 9.2 Hz, 2H), 6.91 (d, J = 9.2 Hz, 2H), 4.79 (d, J = 7.2 Hz, 1 H), 4.06 (app t, J = 3.6 Hz, 1 H), 3.94 (dd, J = 10.4, 6.0 Hz, 1 H), 3.88 (dd, J = 10.4, 4.8 Hz, 1 H), 3.90–3.80 (m, 1 H), 3.85 (s, 3 H), 3.60 (t, J = 5.6 Hz, 1 H), 3.24 (dd, J = 9.6, 7.6 Hz, 1 H), 2.78 (d, J = 4.8 Hz, 1 H), 4.27 (brs, 1 H), 0.92 (s, 9 H), 0.78 (s, 9 H), 0.11 (s, 3 H), 0.10 (s, 3 H), 0.07 (s, 3 H), −0.01 (s, 3 H); 13C NMR (CDCl3, 100 MHz): δ = 164.9, 161.7, 131.9, 130.2, 113.7, 96.6, 75.6, 74.8, 72.6, 68.0, 62.8, 55.3, 25.8, 25.5, 18.2, 17.9, −4.3, −5.3, −5.45, −5.51; IR (film, cm−1): ν = 3419, 2927, 1644, 1605, 1577, 1512, 1249.
3,4-Di-O-Benzyl-1,6-Di-O-tert-Butyldimethylsilyl-2-Deoxy-2-p-Methoxybenzylideneamino-d-Galactopyranoside 53C
A 250 mL RBF was charged with diol 53B (1.17 g, 2.2 mmol, 1.0 equiv.), benzyl bromide (0.78 mL, 6.6 mmol, 3.0 equiv.) and DMF (10 mL). The solution was cooled to −20 °C, and NaH (60%, 0.22 g, 5.5 mmol, 2.5 equiv.) was added in several portions. The reaction was allowed to warm to room temperature and stirred overnight. The reaction mixture was diluted with ethyl acetate, washed with brine (2 × 50 mL), dried over Na2SO4, concentrated in vacuo. The residue was purified by silica gel flash chromatography (hexane/ethyl acetate = 20/1 → 10/1 with 1% Et3N) to afford 53C (1.158 g, 75%) as a viscous oil. 1H NMR (CDCl3, 400 MHz): δ = 8.30 (s, 1 H), 7.70 (d, J = 8.8 Hz, 2 H), 7.45–7.15 (m, 10 H), 6.94 (d, J = 8.8 Hz, 2 H), 4.97 (d, J = 11.6 Hz, 1 H), 4.82 (d, J = 7.2 Hz, 1 H), 4.56 (d, J = 11.6 Hz, 1 H), 4.62–4.53 (m, 2 H), 3.87 (s, 3 H), 3.88–3.83 (m, 1 H), 3.80–3.64 (m, 3 H), 3.60 (dd, J = 10.0, 7.2 Hz, 1 H), 3.49 (t, J = 6.4 Hz, 1 H), 0.91 (s, 9 H), 0.79 (s, 9 H), 0.07 (s, 3 H), 0.06 (s, 6 H), 0.01 (s, 3 H); 13C NMR (CDCl3, 100 MHz): δ = 163.2, 161.4, 138.9, 138.5, 129.8, 129.5, 128.2, 128.1, 128.0, 127.7, 127.4, 113.8, 97.2, 81.3, 75.7, 74.9, 74.3, 72.3, 72.3, 62.3, 55.3, 25.9, 25.6, 18.2, 18.0, −4.1, −5.2, −5.4, −5.5; IR (film, cm−1): ν = 2928, 1847, 1606, 1512, 1248.
3,4-Di-O-Benzyl-2-Deoxy-2-Amino-d-Galactopyranose 53D
A 25 mL RBF was charged with 53C (105 mg, 0.15 mmol, 1.0 equiv.), 6N HCl (0.2 mL, 1.2 mmol, 8.0 equiv.) and acetone (1.0 mL). The solution was stirred at 50 °C overnight, concentrated in vacuo, and the residue was purified by silica gel flash chromatography (CH2Cl2/MeOH = 20/1 → 5/1) to afford 56 mg (95%) of 53D as a viscous oil. 1H NMR (CD3OD, 400 MHz): δ = 7.51–7.24 (m, 10 H), 5.33 (d, J = 3.6 Hz, 1 H), 4.65–4.61 (m, 2 H), 4.59–4.52 (m, 2 H), 4.23–4.18 (m, 1 H), 4.11–4.02 (m, 2 H), 3.72–3.62 (m, 2 H), 3.57 (dd, J = 10.8, 3.6 Hz, 1 H); 13C NMR (CD3OD, 100 MHz): δ = 139.6, 138.6, 129.2, 129.1, 128.9, 128.8, 128.7, 128.4, 91.2, 77.1, 75.6, 73.5, 72.2, 72.1, 61.9, 52.1
1,6-Di-O-Acetyl-3,4-Di-O-Benzyl-2-Deoxy-2-o-Trifluromethylbenzylideneamino-d-Galactopyranoside 53E
A 25 mL RBF was charged with 53D (40 mg, 0.1 mmol, 1.0 equiv.), 1M NaOH (0.12 mL, 0.12 mmol, 1.2 equiv.), and CH2Cl2 (0.5 mL). To this pale yellow solution was then added 2 trifluoromethylbenzylaldehyde (0.02 mL, 0.12 mmol, 1.2 equiv.). The resulting mixture was stirred at room temperature overnight. Then the mixture was diluted with ethyl acetate, washed with brine, dried over Na2SO4, concentrated in vacuo. The crude residue was used directly without further purification. A 25 mL RBF was charged with the crude residue, acetic anhydride (0.15 mL), and pyridine (0.30 mL). After the reaction mixture was stirred at room temperature overnight, it was azeotroped with toluene for three times (3 × 15 mL). The residue was then purified by silica gel flash chromatography (hexane/ethyl acetate = 5/1 → 2/1 with 1% Et3N) to afford 53E (45 mg, 75%) as a viscous oil. 1H NMR (CDCl3, 400 MHz): δ = 8.76 (d, J = 2.4 Hz, 1 H), 8.11 (d, J = 7.6 Hz, 1 H), 7.70 (d, J = 7.6 Hz, 1 H), 7.62–7.47 (m, 2 H), 7.34–7.23 (m, 10 H), 5.85 (d, J = 7.2 Hz, 1 H), 4.99 (d, J = 11.6 Hz, 1 H), 4.67–4.53 (m, 3 H), 4.23 (dd, J = 11.2, 6.8 Hz, 1 H), 4.15 (dd, J = 11.2, 6.4 Hz, 1 H), 3.96–3.88 (m, 4 H), 1.99 (s, 6 H); 13C NMR (CDCl3, 100 MHz): δ = 170.5, 169.1, 161.5, 138.0, 137.7, 133.9, 132.0, 130.4, 129.3, 129.0, 128.4, 128.3, 127.83, 127.77, 127.7, 125.6 (q, JC-F = 5.7 Hz), 124.0 (d, JC-F = 272 Hz), 93.3, 80.7, 74.4, 73.5, 72.6, 71.4, 63.1, 20.8, 20.6; IR (film, cm−1): ν = 2936, 1749, 1644, 1368, 1228.
6-O-Acetyl-3,4-Di-O-Benzyl-2-Deoxy-2-o-Trifluromethylbenzylideneamino-d-Galactopyranose 53F
A 50 mL oven dried Schlenk flask was charged with 53E (500 mg, 0.83 mmol, 1.0 equiv.) and THF (5.0 mL). The solution was cooled to 0 °C, and a solution of NH3 in methanol (7 N, 3.6 mL, 25.0 mmol, 30 equiv.) was added. The resulting mixture was warmed to room temperature and stirred. When the reaction was complete as monitored by TLC, the mixture was evaporated and purified by flash chromatography on silica gel (hexane/ethyl acetate = 2/1 → 1/2 with 1% Et3N) to afford 53F (300 mg, 65%) as a viscous oil. 1H NMR (CDCl3, 400 MHz): δ = 8.77 (d, J = 2.4 Hz, 1 H), 6.19 (d, J = 8.0 Hz, 1 H), 7.70 (d, J = 7.2 Hz, 1 H), 7.63–7.52 (m, 2H), 7.38–7.21 (m, 10 H), 4.99 (d, J = 11.6 Hz, 1 H), 4.94 (d, J = 7.2 Hz, 1 H), 4.68–4.50 (m, 3 H), 4.32–4.11 (m, 2 H), 3.84–3.68 (m, 4 H), 2.02 (s, 3 H); IR (film, cm−1): ν = 3423, 2894, 1742, 1644, 1455, 1314.
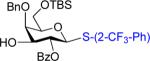
6-O-Acetyl-3,4-Di-O-Benzyl-2-Deoxy-2-o-Trifluromethylbenzylideneamino-dGalactopyranosyl N-Phenyl Trifluoroacetimidate 53
Viscous oil: 377 mg, 99%;1H NMR (CDCl3, 400 MHz): δ = 8.82 (d, J = 1.2 Hz, 1 H), 8.16 (d, J = 7.6 Hz, 1 H), 7.73 (d, J = 7.6 Hz, 1 H), 7.64–7.53 (m, 2 H), 7.37–7.21 (m, 12 H), 7.06 (t, J = 3.6 Hz, 1 H), 6.76 (d, J = 7.6 Hz, 2 H), 5.91 (brs, 1 H), 5.01 (d, J = 11.6 Hz, 1 H), 4.73–4.57 (m, 3 H), 4.28 (dd, J = 11.2, 6.8 Hz, 1 H), 4.17 (dd, J = 11.2, 5.6 Hz, 1 H), 4.07–4.01 (m, 1 H), 3.90–3.62 (m, 3 H), 1.97 (s, 3 H); 13C NMR (CDCl3, 100 MHz): δ = 170.5, 161.9, 143.6, 137.9, 137.6, 133.9, 131.9, 130.5, 128.5 (q, JC-F = 31.0 Hz), 128.6, 128.41, 128.38, 128.36, 127.9, 127.8, 127.7, 125.7 (q, JC-F = 5.6 Hz), 124.1, 124.0 (d, JC-F = 272 Hz), 119.2, 96.3, 80.5, 74.5, 73.6, 72.6, 71.5, 71.3, 63.0, 20.7; IR (film, cm−1): ν = 2890, 1744, 1598, 1368, 1315; HRMS (ESI): calc. for C38H35F6N2O6 (M+H): 729.2399; found: 729.2405.
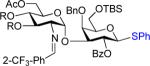
6-O-Acetyl-3,4-Di-O-Benzyl-2-Deoxy-2-o-Trifluromethylbenzylideneamino-d-Glucopyranosyl N-Phenyl Trifluoroacetimidate 32
Viscous oil: 7.76 g, 98%, β only; 1H NMR (CDCl3, 400 MHz): δ = 8.76 (s, 1 H), 8.20 (d, J = 7.6 Hz, 1 H), 7.73 (d, J = 7.6 Hz, 1 H), 7.63–7.54 (m, 2 H), 7.37–7.06 (m, 13 H), 6.83–6.74 (m, 2 H), 5.92 (brs, 1 H), 4.86 (d, J = 10.8 Hz, 1 H), 4.73–4.57 (m, 3 H), 4.40–4.26 (m, 2 H), 4.06–3.94 (m, 1 H), 3.75–3.58 (m, 3 H), 2.05 (s, 3 H). 13C NMR (CDCl3, 100 MHz): δ = 170.7, 161.7, 143.3, 137.5, 137.4, 133.4, 132.0, 130.7, 128.6, 128.5, 128.3, 128.14, 128.09, 127.9, 127.8, 126.2, 125.7 (q, JC-F = 5.6 Hz), 124.3, 124.0 (d, JC-F = 272 Hz), 119.2, 95.6, 83.2, 76.1, 75.2, 75.1, 73.9, 73.0, 62.6, 20.8. IR (film, cm−1): ν = 3032, 2978, 1738, 1719, 1644, 1488, 1313. HRMS (ESI): calc. for C38H34F6N2O6Na (M+Na): 751.2219; found: 751.2217.
3,4,6-Tri-O-Acetyl-1-tert-Butyldimethylsilyl-2-Deoxy-2-p-Methoxybenzylideneamino-d-Glucopyranoside 33B
A 250 mL oven dried RBF was charged with 33A (13.0 g, 30.7 mmol, 1.0 equiv.),2 TBSCl (5.10 g, 33.8 mmol, 1.1 equiv.), imidazole (4.2 g, 61.4 mmol, 2.0 equiv.) and DMF (120 mL). The resulting mixture was stirred at room temperature overnight. The reaction mixture was diluted with ethyl acetate, washed with brine (2 × 50 mL), concentrated in vacuo. The residue was purified by silica gel flash chromatography (hexane/ethyl acetate = 5/1 → 3/1 with 1% Et3N) to afford 33B (16.4 g, 99%) as a viscous oil. 1H NMR (CDCl3, 400 MHz): δ = 8.13 (s, 1 H), 7.63 (d, J = 8.8 Hz, 2 H), 6.89 (d, J = 8.8 Hz, 2 H), 5.45 (t, J = 9.6, 1 H), 5.06 (app t, J = 9.6 Hz, 1 H), 4.93 (d, J = 7.6 Hz, 1 H), 4.28 (dd, J = 12.0, 6.0 Hz, 1 H), 4.18–3.97 (m, 2 H), 3.84 (s, 3 H), 3.24 (dd, J = 7.6, 10.0 Hz, 1 H), 2.08 (s, 3 H), 2.03 (s, 3 H), 1.88 (s, 3 H), 0.79 (s, 9 H), 0.06 (s, 3 H), 0.00 (s, 3 H).
1-tert-Butyldimethylsilyl-2-Deoxy-2-p-Methoxybenzylideneamino-d-Glucopyranoside 33C
A 250 mL oven dried RBF was charged with 33B (6.5 g, 12.0 mmol, 1.0 equiv.), sodium methoxide (0.52 g, 9.6 mmol, 0.8 equiv.), and CH2Cl2/MeOH (50 mL/100 mL). The resulting solution was stirred at room temperature. When the reaction mixture was complete as monitored by TLC, it was evaporated and then purified by flash chromatography on silica gel (ethyl acetate → CH2Cl2/MeOH = 5/1) to afford 33C (4.80 g, 96%) as a viscous oil. 1H NMR (CD3OD, 400 MHz): δ = 8.21 (s, 1 H), 7.72 (d, J = 8.8 Hz, 2 H), 6.98 (d, J = 8.8 Hz, 2 H), 4.88 (d, J = 7.2 Hz, 1 H), 3.92–3.86 (m, 1 H), 3.85 (s, 3 H), 3.76–3.72 (m, 1 H), 3.47–3.38 (m, 2 H), 2.97 (dd, J = 9.6, 7.2 Hz, 1 H), 0.78 (s, 9 H), 0.09 (s, 3 H), 0.02 (s, 3 H).
3,4,6-Tri-O-Benzyl-1-tert-Butyldimethylsilyl-2-Deoxy-2-p-Methoxybenzylideneamino-d Glucopyranoside 33D
A 250 mL RBF was charged with 33C (12.3 g, 30.0 mmol, 1.0 equiv.), benzyl bromide (16.2 mL, 135 mmol, 4.5 equiv.) and DMF (100 mL). The solution was cooled to − 20 °C, and NaH (60%, 5.40 g, 135 mmol, 4.5 equiv.) was added in several portions. The mixture was warmed to room temperature and stirred overnight. The reaction mixture was diluted with ethyl acetate (100 mL), washed with brine (2 × 30 mL), dried over Na2SO4, concentrated in vacuo. The residue was purified by silica gel flash chromatography (hexane/ethyl acetate = 20/1 → 10/1 → 5/1 with 1% Et3N) to afford 33D (15.6 g, 76%) as a viscous oil. 1H NMR (CDCl3, 400 MHz): δ = 8.24 (s, 1 H), 7.68 (d, J = 8.8 Hz, 2 H), 7.36–7.27 (m, 12 H), 7.17–7.09 (m, 3 H), 6.94 (d, J = 8.8 Hz, 1 H), 4.91–4.87 (m, 2 H), 4.68–4.55 (m, 5 H), 3.93 (t, J = 9.2 Hz, 1 H), 3.86 (s, 3 H), 3.80–3.62 (m, 4 H), 3.23 (dd, J = 9.2, 7.2 Hz, 1 H), 0.81 (s, 9 H), 0.10 (s, 3 H), 0.02 (s, 3 H).
3,4,6-Tri-O-Benzyl-2-Deoxy-2-Amino-d-Glucopyranose 33E
A 250 mL RBF was charged with 33D (15.1 g, 22.2 mmol, 1.0 equiv.), 6N HCl (18.5 mL, 110 mmol, 5.0 equiv.) and acetone (200 mL). The solution was stirred at 40 °C. When the reaction mixture was complete as monitored by TLC, it was then evaporated and purified by flash chromatography on silica gel (hexane/ethyl acetate = 2/1 → CH2Cl2/MeOH = 10/1) to afford 33E (8.17 g, 82%) as a viscous oil. 1H NMR (CDCl3+CD3OD, 400 MHz): δ = 7.25–7.18 (m, 13 H), 7.03–6.98 (m, 2 H), 5.38 (s, 1 H), 4.86 (d, J = 10.4 Hz, 1 H), 4.69 (d, J = 10.4 Hz, 1 H), 4.62 (d, J = 10.4 Hz, 1 H), 4.47–4.36 (m, 2 H), 3.70–3.53 (m, 4 H), 3.10 (d, J = 7.2 Hz, 1 H); 13C NMR (CDCl3+CD3OD, 100 MHz): δ = 137.3, 137.1, 137.0, 128.21, 128.17, 127.8, 127.7, 127.5, 127.4, 86.9, 78.5, 74.9, 74.5, 73.2, 69.9, 67.8, 54.0; IR (film, cm−1): ν = 3435, 2978, 1585, 1487.
3,4,6-Tri-O-Benzyl-2-Deoxy-2-o-Trifluoromethylbenzylideneamino-d-Glucopyranose 33F
A 250 mL RBF was charged with 33E (4.50 g, 10.0 mmol, 1.0 equiv.), 2-trifluoromethylbenzylaldehyde (1.5 mL, 11.0 mmol, 1.1 equiv.), pyridine (8.1 mL, 100 mmol, 100 equiv.) and CH2Cl2 (100 mL). The resulting solution was stirred under reflux overnight. The reaction mixture was azeotroped with toluene and purified by flash chromatography on silica gel (Hexane/ethyl acetate = 2/1 ~ 1/2) to afford 3.11 g (52%) of 33F as a viscous oil. 1H NMR (CDCl3, 400 MHz): δ = 8.71 (d, J = 2.0 Hz, 1 H), 8.21 (d, J = 6.0 Hz, 1 H), 7.69 (d, J = 6.0 Hz, 1 H), 7.58–7.47 (m, 2 H), 7.35–7.05 (m, 15 H), 5.01 (d, J = 8.0 Hz, 1 H), 4.82 (d, J = 10.8 Hz, 1 H), 4.70–4.47 (m, 5 H), 3.92 (t, J = 8.0 Hz, 1 H), 3.83–3,65 (m, 3 H), 3.60 (dd, J = 9.6, 3.6 Hz, 1 H), 3.34 (dd, J = 8.0, 9.6 Hz, 1 H); IR (film, cm−1): ν = 3403, 2820, 1640, 1454, 1359, 1312.
3,4,6-Tri-O-Benzyl-2-Deoxy-2-o-Trifluoromethylbenzylideneamino-d-GlucopyranosylN-Phenyl Trifluoroacetimidate 3321
Viscous oil: 3.95 g, 98%, α:β = 1:3; 33α:1H NMR (CDCl3, 400 MHz): δ = 8.77 (s, 1 H), 8.35 (d, J = 7.6 Hz, 1 H), 7.75 (d, J = 7.6 Hz, 1 H), 7.67–7.55 (m, 2 H), 7.43–6.55 (m, 20 H), 6.49 (brs, 1 H), 4.89 (d, J = 10.8 Hz, 1 H), 4.77–4.68 (m, 2 H), 4.65–4.53 (m, 3 H), 4.32–4.20 (m, 2 H), 3.96–3.85 (m, 2 H), 3.83–3.72 (m, 2 H); 13C NMR (CDCl3, 100 MHz): δ = 160.6, 137.9, 137.8, 137.7, 133.4, 132.0, 130.7, 129.4 (q, JC-F = 31.0 Hz), 128.5, 128.44, 128.38, 128.3, 128.2, 128.0, 127.92, 127.88, 127.85, 127.7, 127.6, 125.6 (q, JC-F =5.6 Hz), 123.9 (d, JC-F = 273 Hz), 95.7, 80.2, 75.3, 74.2, 73.8, 73.5, 60.4; IR (film, cm−1): ν = 2876, 1667, 1594, 1488, 1313; HRMS (ESI): calc. for C43H38F6N2O5Na (M+Na): 799.2583; found: 799.2598. 33β:1H NMR (CDCl3, 400 MHz): δ = 8.79 (s, 1 H), 8.22 (d, J = 7.6 Hz, 1 H), 7.75 (d, J = 7.6 Hz, 1 H), 7.63–7.54 (m, 2 H), 7.43–7.05 (m, 18 H), 6.85–6.74 (m, 2 H), 6.07 (brs, 1 H), 4.89 (d, J = 10.8 Hz, 1 H), 4.77–4.56 (m, 5 H), 4.10–3.63 (m, 6 H); 13C NMR (CDCl3, 100 MHz): δ = 161.5, 137.9, 137.8, 137.7, 135.2, 133.5, 131.9, 130.6, 129.5 (q, JC-F = 31.0 Hz), 129.3, 128.6, 128.4, 128.3, 128.2, 127.9, 127.8, 127.6, 126.2, 125.7 (q, JC-F = 5.5 Hz), 124.1, 123.9 (d, JC-F = 273 Hz), 95.8, 83.0, 76.2, 76.0, 75.2, 75.1, 73.4, 60.4; IR (film, cm−1): ν = 3031, 2865, 1644, 1597, 1488, 1314; HRMS (ESI): calc. for C43H38F6N2O5Na (M+Na): 799.2584; found: 799.2598.
General Glycosylation Procedure Using Ni(4-F-PhCN)4(OTf)2: Formation of Disaccharide 5 and Transfer Product 7
A 10 mL oven dried Schlenk flask was charged with donor 10 (31 mg, 0.05 mmol, 1.0 equiv), thioglycoside acceptor 3 (53 mg, 0.10 mmol, 2.0 equiv), and CH2Cl2 (0.3 mL). Then a 0.25 mL of preformed solution of Ni(4-F-PhCN)4(OTf)2, which was generated in situ from a reaction of Ni(4-F-PhCN)4Cl2 (12 mg, 0.02 mmol, 10 mol%) and AgOTf (11 mg, 0.4 mmol, 20 mol%) in dichloromethane (1.0 mL) for 30 min, was added to the solution. The resulting mixture was stirred at 35 °C overnight. When the reaction was complete as monitored by TLC (toluene/acetonitrile = 4/1), the mixture was evaporated, and purified by flash chromatography on silica gel (hexane/ethyl acetate = 9/1 → 5/1 → 3/2 with 1% Et3N) to afford disaccharide 5 (28 mg, 58%, α only) and thioglycoside 7 (4 mg, 14%, α only) as viscous oil.
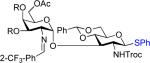
Phenyl 3,4,6-Tri-O-Acetyl-2-Deoxy-2-o-Trifluoromethylbenzylideneamino-α-d-Glucopyranosyl-(1→3)-4,6-O-Benzylidene-2-Deoxy-2-(2,2,2-Trichloroethyl)amino-1-Thiol-β-d-Glucopyranoside 5
[α]24D = 28.2 (c = 2.1, CH2Cl2); 1H NMR (CDCl3, 400 MHz): δ = 8.43 (d, J = 2.0 Hz, 1 H), 8.22 (d, J = 7.6 Hz, 1 H), 7.62 (d, J = 7.6 Hz, 1 H), 7.56–7.33 (m, 7 H)., 7.23–7.16 (m, 1 H), 7.14–7.03 (m, 2 H), 6.93 (d, J = 7.2 Hz, 2 H), 5.65–5.51 (m, 2 H), 5.37 (d, J = 3.6 Hz, 1 H), 5.19 (s, 1 H), 5.09 (t, J = 5.6 Hz, 2 H), 4.98 (d, J = 12.0 Hz, 1 H), 4.61 (d, J = 12.0 Hz, 1 H), 4.38–4.26 (m, 4 H), 4.06 (dd, J = 12.0, 2.0 Hz, 1 H), 3.92–3.65 (m, 3 H), 3.62 (dd, J = 10.4, 3.6 Hz, 1 H), 3.56–3.49 (m, 1 H), 2.11 (s, 3 H), 2.02 (s, 3 H), 1.82 (s, 3 H); 13C NMR (CDCl3, 100 MHz): δ = 170.7, 169.7, 169.6, 160.4, 153.8, 136.3, 132.4, 131.9, 130.7, 129.1, 128.9, 128.3, 128.2, 127.9, 126.3, 125.5, 101.5, 99.0, 95.2, 87.1, 82.0, 76.7, 74.6, 72.3, 70.7, 70.0, 68.7, 68.5, 67.8, 62.1, 55.7, 20.8, 20.7, 20.3; IR (film, cm−1): ν = 3335, 2872, 1746, 1643, 1523, 1368, 1314, 1226; HRMS (ESI): calc. for C42H43Cl3F3N2O13S (M+H): 977.1504; found: 977.1505.
Phenyl 3,4,6-Tri-OAcetyl-2-Deoxy-2-o-Trifluoromethylbenzylideneamino-1-Thiol-α-d-Glucopyranoside 7
1H NMR (CDCl3, 400 MHz):22 δ = 8.66 (d, J = 2.0 Hz, 1 H), 8.27 (d, J = 7.6 Hz, 1 H), 7.70–7.47 (m 5 H), 7.33–7.22 (m, 2 H), 5.72–5.62 (m, 2 H), 5.14 (app t, J = 9.6 Hz, 1 H), 4.72–4.65 (m, 1 H), 4.30 (dd, J = 5.6, 12.0 Hz, 1 H), 4.02 (dd, J = 5.6, 10.0 Hz, 1 H), 3.97 (dd, J = 2.0, 12.0 Hz, 1 H), 2.05 (s, 3 H), 2.00 (s, 3 H), 1.89 (s, 3 H). 13C NMR (CDCl3, 100 MHz): δ = 170.6, 169.9, 169.7, 160.3, 133.2, 132.3, 131.7, 130.9, 129.2, 129.0, 127.3, 125.4, 86.3, 72.2, 71.7, 68.6, 68.4, 62.1, 20.7, 20.6, 20.4. IR (film, cm−1): ν = 2932954, 1744, 1643, 1366, 1314, 1221. HRMS (ESI): calc. for C26H27F3NO7S (M+H): 554.1460; found: 554.1473.
Phenyl 3,4,6-Tri-O-Acetyl-2-Deoxy-2-p-Trifluoromethylbenzylideneamino-α-d-Glucopyranosyl-(1→3)-4,6-O-Benzylidene-2-Deoxy-2-(2,2,2-Trichloroethyl)amino-1-Thiol-β-d-Glucopyranoside 4
Viscous oil: 16 mg, 33%, α:β = 21:1; 1H NMR (CDCl3, 400 MHz): δ = 8.17 (s, 1 H), 7.48–7.31 (m, 11 H), 7.16–7.14 (m, 2 H), 6.63–6.60 (m, 1 H), 5.65 (t, J = 9.6 Hz, 1 H), 5.55 (app t, J = 9.2 Hz, 1 H), 5.36 (d, J = 4.0 Hz, 1 H), 5.18–4.94 (m, 4 H), 4.60 (d, J = 12.0 Hz, 1 H), 4.42–4.03 (m, 5 H), 3.72–3.45 (m, 5 H), 2.12 (s, 3 H), 2.04 (s, 3 H), 1.83 (s, 3 H);13C NMR (CDCl3, 100 MHz): δ = 170.9, 170.8, 170.7, 132.7, 132.5, 129.2, 128.4, 128.0, 126.0, 125.7, 100.5, 99.6, 95.2, 77.2, 74.6, 71.0, 70.1, 70.0, 68.6, 68.5, 20.9, 20.7, 20.6; IR (film, cm−1): ν = 3343, 2824, 1744, 1648, 1532, 1368, 1323, 1230; HRMS (ESI): calc. for C42H43Cl3F3N2O13S (M+H): 977.1504; found: 977.1512.
Phenyl 3,4,6-Tri-O-Acetyl-2-Deoxy-2-p-Trifluoromethylbenzylideneamino-1-Thiol-α-d-Glucopyranoside 6
Viscous oil: 9 mg, 33%, α only; 1H NMR (CDCl3, 400 MHz): δ = 8.34 (s, 1 H), 7.90 (d, J = 8.0 Hz, 2 H), 7.69 (d, J = 8.0 Hz, 2 H), 7.48–7.45 (m, 2 H), 7.33–7.24 (m, 3 H), 5.72–5.64 (m, 2 H), 5.13 (app t, J = 10.0 Hz, 1 H), 4.75–4.67 (m, 1 H), 4.32 (dd, J = 12.0, 5.2 Hz, 1 H), 4.00–3.93 (m, 2 H), 2.06 (s, 3 H), 2.00 (s, 3 H), 1.88 (s, 3 H); 13C NMR (CDCl3, 100 MHz): δ = 170.6, 170.2, 169.5, 162.4, 133.1, 131.8, 131.7, 129.0, 127.4, 125.7 (q, JC-F = 5.5 Hz), 86.5, 72.1, 71.9, 68.8, 68.4, 62.1, 20.74, 20.70, 20.6; IR (film, cm−1): ν = 2923, 1748, 1647, 1454, 1370, 1324, 1234; HRMS (ESI): calc. for C26H27F3NO7S (M+H): 554.1460; found: 554.1463.
Ethyl 3,4,6-Tri-O-Acetyl-2-Deoxy-2-o-Trifluoromethylbenzylideneamino-α-d-Glucopyranosyl-(1→3)-4,6-O-Benzylidene-2-Deoxy-2-(2,2,2-Trichloroethyl)amino-1-Thiol-β-d-Glucopyranoside 18
Viscous oil: 32 mg, 43%, α:β = 10:1; 1H NMR (CDCl3, 400 MHz): δ = 8.42 (d, J = 2.0 Hz, 1 H), 8.23 (d, J = 7.6 Hz, 1 H), 7.64–7.33 (m, 4 H), 7.22–7.07 (m, 1 H), 7.11–7.04 (m, 2 H), 6.91 (d, J = 7.2 Hz, 2 H), 5.76 (t, J = 10.0 Hz, 1 H), 5.49 (d, J = 8.8 Hz, 1 H), 5.18 (s, 1 H), 5.09 (app t, J = 10.0 Hz, 2 H), 5.02 (d, J = 12.0 Hz, 1 H), 4.57 (d, J = 12.0 Hz, 1 H), 4.40–4.23 (m, 3 H), 4.19 (t, J = 9.2 Hz, 1 H), 4.10 (dd, J = 12.0, 2.0 Hz, 1 H), 3.85–3.73 (m, 2 H), 3.71 (t, J = 10.0 Hz, 1 H), 3.62 (dd, J = 10.0, 4.0 Hz, 1 H), 3.51 (td, J = 9.6, 4.8 Hz, 1 H), 2.76 (q, J = 7.2 Hz, 2 H), 2.12 (s, 3 H), 2.03 (s, 3 H), 1.83 (s, 3 H), 1.27 (t, J = 7.2 Hz, 3 H); 13C NMR (CDCl3, 100 MHz): δ = 170.7, 169.8, 169.6, 160.4, 153.9, 136.3, 131.9, 130.8, 129.0, 128.3, 128.0, 126.2, 125.5, 101.5, 99.1, 95.2, 85.1, 82.2, 77.2, 74.6, 72.3, 70.7, 70.1, 68.6, 67.8, 62.3, 55.6, 24.3, 20.9, 20.8, 20.4, 14.8; IR (film, cm−1): ν = 3332, 2872, 1746, 1643, 1527, 1368, 1314, 1227; HRMS (ESI): calc. for C38H43Cl3F3N2O13S (M+H): 929.1504; found: 929.1516.
Ethyl 3,4,6-Tri-O-Acetyl-2-Deoxy-2-o-Trifluoromethylbenzylideneamino-1-Thiol-α-d-Glucopyranoside 25
Viscous oil: 11 mg, 22%, α only; 1H NMR (CDCl3, 400 MHz):22 δ = 8.61 (d, J = 2.0 Hz, 1 H), 8.21 (d, J = 7.6 Hz, 1 H), 7.67 (d, J = 7.6 Hz, 1 H), 7.62–7.53 (m, 2 H), 5.58 (t, J = 9.6 Hz, 1 H), 5.41 (d, J = 5.6 Hz, 1 H), 5.13 (app t, J = 10.0 Hz, 1 H), 4.64–4.57 (m, 1 H), 4.38 (dd, J = 12.0, 4.8 Hz, 1 H), 4.12 (dd, J = 12.0, 2.0 Hz, 1 H), 3.95 (dd, J = 10.0, 5.6 Hz, 1 H), 2.66–2.48 (m, 2 H), 2.11 (s, 3 H), 2.04 (s, 3 H), 1.86 (s, 3 H), 1.30 (t, J = 7.2 Hz, 3 H); 13C NMR (CDCl3, 100 MHz): δ = 170.7, 170.0, 169.7, 160.0, 133.2, 132.3, 130.8, 129.1, 125.4 (q, JC-F = 5.5 Hz), 83.5, 72.2, 71.8, 68.8, 67.7, 62.2, 23.6, 20.8, 20.7, 20.4, 14.4; IR (film, cm−1): ν = 2930, 1748, 1644, 1453, 1368, 1315, 1227; HRMS (ESI): calc. for C22H26F3NO7S (M+H): 506.1460; found: 506.1456.
Naphthyl 3,4,6-Tri-O-Acetyl-2-Deoxy-2-o-Trifluoromethylbenzylideneamino-α-d-Glucopyranosyl-(1→3)-4,6-O-Benzylidene-2-Deoxy-2-(2,2,2-Trichloroethyl)amino-1-Thiol-β-d-Glucopyranoside 19
Viscous oil: 30 mg, 59%, α only; [α]24D = 6.1 (c = 1.0, CH2Cl2); 1H NMR (CDCl3, 400 MHz): δ = 8.53–8.47 (m,1 H), 8.40 (d, J = 2.0 Hz, 1 H), 8.20 (d, J = 7.6 Hz, 1 H), 7.92–7.83 (m, 3 H), 7.64–7.43 (m, 5 H), 7.18–7.13 (m, 1 H), 7.08–7.03 (m, 2 H), 6.89 (d, J = 7.2 Hz, 2 H), 5.63 (d, J = 8.4 Hz, 1 H), 5.54 (t, J = 9.6 Hz, 1 H), 5.34 (d, J = 4.0 Hz, 1 H), 5.17 (s, 1 H), 5.13–5.05 (m, 2 H), 4.99 (d, J = 12.0 Hz, 1 H), 4.62 (d, J = 12.0 Hz, 1 H), 4.34–4.23 (m, 4 H), 4.08–4.02 (m, 1 H), 3.81–3.65 (m, 3 H), 3.60 (dd, J = 10.4, 4.0 Hz, 1 H), 3.46 (td, J = 9.6, 4.8 Hz, 1 H), 2.10 (s, 3 H), 2.02 (s, 3 H), 1.82 (s, 3 H); 13C NMR (CDCl3, 100 MHz): δ = 170.7, 169.8, 169.7, 160.5, 153.7, 136..3, 134.1, 133.2, 131.9, 130.8, 129.7, 128.9, 128.7, 128.3, 127.9, 126.9, 126.4, 125.7, 125.5, 101.5, 99.1, 95.1, 82.1, 77.2, 74.7, 72.3, 70.6, 70.0, 68.6, 67.8, 62.1, 55.8, 20.8, 20.7, 20.4; IR (film, cm−1): ν = 3335, 2965, 1744, 1643, 1532, 1368, 1314, 1225; HRMS (ESI): calc. for C46H45Cl3F3N2O13S (M+H): 1027.1660; found: 1027.1650.
Naphthyl 3,4,6-Tri-O-Acetyl-2-Deoxy-2-o-Trifluoromethylbenzylideneamino-1-Thiol-α-d-Glucopyranoside 26
Viscous oil: 3 mg, 10%, α only; 1H NMR (CDCl3, 400 MHz):22 δ = 8.71 (d, J = 2.0 Hz, 1 H), 8.57–8.46 (m, 2 H), 7.86–7.26 (m, 9 H), 5.77 (t, J = 9.6 Hz, 1 H), 5.65 (d, J = 5.6 Hz, 1 H), 4.83–4.74 (m, 1 H), 4.29 (dd, J = 12.0, 4.8 Hz, 1 H), 4.05 (dd, J = 10.0, 5.6 Hz, 1 H), 3.90–3.83 (m, 1 H), 3.63–3.52 (m, 1 H), 2.07 (s, 3 H), 1.94 (s, 3 H), 1.92 (s, 3 H); 13C NMR (CDCl3, 100 MHz): δ = 170.6, 169.8, 147.8, 134.1, 133.1, 132.3, 130.9, 129.14, 129.09, 128.9, 128.3, 127.2, 126.8, 126.2, 125.1, 124.1, 87.0, 74.1, 72.2, 71.8, 68.8, 62.1, 20.7, 20.5; IR (film, cm−1): ν = 2924, 1745, 1644, 1439, 1369, 1313, 1221; HRMS (ESI): calc. for C30H29F3NO7S (M+H): 604.1617; found: 604.1615.
4-Methyphenyl 3,4,6-Tri-O-Acetyl-2-Deoxy-2-o-Trifluoromethylbenzylideneamino-α-d-Glucopyranosyl-(1→3)-4,6-O-Benzylidene-2-Deoxy-2-(2,2,2-Trichloroethyl)amino-1-Thiol-β-d-Glucopyranoside 20
Viscous oil: 37 mg, 38%, α:β =11:1; 1H NMR (CDCl3, 400 MHz): δ = 8.42 (d, J = 2.0 Hz, 1 H), 8.20 (d, J = 7.2 Hz, 1 H), 7.63–7.52 (m, 3 H), 7.39 (d, J = 8.0 Hz, 2 H), 7.18–7.13 (m, 3 H), 7.07–7.04 (m, 2 H), 6.91 (d, J = 8.0 Hz, 2 H), 5.60–5.53 (m, 2 H), 5.36 (d, J = 4.0 Hz, 1 H), 5.17 (s, 1 H), 5.15–5.04 (m, 2 H), 5.00 (d, J = 12.0 Hz, 1 H), 4.59 (d, J = 12.0 Hz, 1 H), 4.36–4.23 (m, 4 H), 4.07–4.04 (m, 1 H), 3.78–3.66 (m, 2 H), 3.61 (dd, J = 10.4, 4.0 Hz, 1 H), 3.54–3.48 (m, 1 H), 2.36 (s, 3 H), 2.11 (s, 3 H), 2.02 (s, 3 H), 1.82 (s, 3 H); 13C NMR (CDCl3, 100 MHz): δ = 170.7, 169.8, 169.7, 160.5, 153.7, 138.6, 136.3, 133.2, 130.8, 129.9, 129.0, 128.3, 128.0, 125.5, 101.5, 99.0, 95.2, 82.1, 77.2, 74.6, 72.3, 70.6, 70.0, 68.6, 67.8, 62.1, 55.6, 21.2, 20.8, 20.7, 20.4; IR (film, cm−1): ν = 3383, 2958, 1746, 1642, 1539, 1368, 1314, 1224; HRMS (ESI): calc. for C43H45Cl3F3N2O13S (M+H): 991.1660; found: 991.1673.
4-Methylphenyl 3,4,6-Tri-O-Acetyl-2-Deoxy-2-o-Trifluoromethylbenzylideneamino-1-Thiol-α-d-Glucopyranoside 27
Viscous oil: 11 mg, 20%, α only; 1H NMR (CDCl3, 400 MHz): δ = 8.65 (d, J = 2.0 Hz, 1 H), 8.28 (d, J = 8.0 Hz, 1 H), 7.69 (d, J = 8.0 Hz, 1 H), 7.64–7.53 (m, 2 H), 7.38 (d, J = 8.0 Hz, 2 H), 7.11 (d, J = 8.0 Hz, 2 H), 5.66 (t, J = 9.6 Hz, 1 H), 5.62 (d, J = 5.6 Hz, 1 H), 5.14 (app t, J = 10.0 Hz, 1 H), 4.73–4.68 (m, 1 H), 4.30 (dd, J = 12.0, 4.8 Hz, 1 H), 4.01 (dd, J = 10.0, 5.6 Hz, 1 H), 3.97 (dd, J = 12.0, 2.0 Hz, 1 H), 2.32 (s, 3 H), 2.06 (s, 3 H), 2.01 (s, 3 H), 1.89 (s, 3 H); 13C NMR (CDCl3, 100 MHz): δ = 170.6, 170.0, 169.7, 137.6, 132.5, 130.9, 129..8, 129.2, 129.1, 86.7, 72.2, 71.7, 68.8, 68.2, 62.2, 21.1, 20.8, 20.7, 20.4; IR (film, cm−1): ν = 2924, 1748, 1643, 1492, 1369, 1315, 1228; HRMS (ESI): calc. for C27H29F3NO7S (M+H): 568.1617; found: 568.1616.
4-Methoxyphenyl 3,4,6-Tri-O-Acetyl-2-Deoxy-2-o-Trifluoromethylbenzylideneamino-α-d-Glucopyranosyl-(1→3)-4,6-O-Benzylidene-2-Deoxy-2-(2,2,2-Trichloroethyl)amino-1-Thiol-β-d-Glucopyranoside 21
Viscous oil: 37 mg, 38%, α:β =11:1; 1H NMR (CDCl3, 400 MHz): δ = 8.41 (d, J = 2.0 Hz, 1 H), 8.20 (d, J = 7.2 Hz, 1 H), 7.63–7.44 (m, 5 H), 7.18–7.13 (m, 1 H), 7.09–7.04 (m, 2 H), 6.92–6.84 (m, 4 H), 5.62–5.52 (m, 2 H), 5.35 (d, J = 4.0 Hz, 1 H), 5.17 (s, 1 H), 5.10 (app t, J = 10.0 Hz, 1 H), 5.03–4.93 (m, 2 H), 4.62 (d, J = 12.0 Hz, 1 H), 4.35–4.22 (m, 4 H), 4.09–4.02 (m, 1 H), 3.83 (s, 3 H), 3.81–3.67 (m, 3 H), 3.61 (dd, J = 4.0, 10.4 Hz, 1 H), 3.53–3.44 (m, 1 H), 2.11 (s, 3 H), 2.02 (s, 3 H), 1.82 (s, 3 H); 13C NMR (CDCl3, 100 MHz): δ = 170.7, 169.8, 169.7, 160.2, 136.3, 135.9, 131.8, 130.7, 128.9, 128.3, 127.9, 127.1, 125.5, 121.6, 114.7, 101.5, 99.0, 95.2, 87.1, 82.0, 77.2, 76.4, 74.6, 72.3, 70.6, 70.0, 68.6, 67.8, 62.1, 55.5, 55.3, 20.8, 20.7, 20.4; IR (film, cm−1): ν = 3342, 2958, 1745, 1642, 1539, 1493, 1368, 1314, 1226; HRMS (ESI): calc. for C43H45Cl3F3N2O14S (M+H): 1007.1609; found: 1007.1597.
4-Methoxyphenyl 3,4,6-Tri-O-Acetyl-2-Deoxy-2-o-Trifluoromethylbenzylideneamino-1-Thiol-α-d-Glucopyranoside 28
Viscous oil: 17 mg, 30%, α only; 1H NMR (CDCl3, 400 MHz):22 δ = 8.65 (d, J = 2.0 Hz, 1 H), 8.32–8.25 (m, 1 H), 7.72–7.54 (m, 3 H), 7.42 (d, J = 8.8 Hz, 2 H), 6.84 (d, J = 8.8 Hz, 2 H), 5.65 (t, J = 9.6 Hz, 1 H), 5.52 (d, J = 5.6 Hz, 1 H), 5.14 (app t, J = 6.0 Hz, 1 H), 4.77–4.71 (m, 1 H), 4.31 (dd, J = 12.0, 4.8 Hz, 1 H), 4.03–3.95 (m, 2 H), 3.79 (s, 3 H), 2.06 (s, 3 H), 2.03 (s, 3 H), 1.89 (s, 3 H); 13C NMR (CDCl3, 100 MHz): δ = 170.7, 169.9, 169.7, 160.2, 159.6, 134.9, 133.2, 132.3, 130.9, 129.2, 129.0, 125.4 (q, JC-F = 5.5 Hz), 124.1, 123.0, 114.6, 87.3, 72.2, 68.8, 68.1, 63.1, 60.9, 55.3, 20.7, 20.5, 20.4; IR (film, cm−1): ν = 2934, 1747, 1644, 1494, 1369, 1315, 1227; HRMS (ESI): calc. for C27H29F3NO8S (M+H): 584.1566; found: 584.1567.
4-Fluorophenyl 3,4,6-Tri-O-Acetyl-2-Deoxy-2-o-Trifluoromethylbenzylideneamino-α-d-Glucopyranosyl-(1→3)-4,6-O-Benzylidene-2-Deoxy-2-(2,2,2-Trichloroethyl)amino-1-Thiol-β-d-Glucopyranoside 22
Viscous oil: 23 mg, 58%, α:β =20:1; 1H NMR (CDCl3, 400 MHz): δ = 8.44 (d, J = 2.0 Hz, 1 H), 8.21 (d, J = 7.2 Hz, 1 H), 7.62–7.50 (m, 5 H), 7.21–7.17 (m, 1 H), 7.10–7.02 (m, 4 H), 6.92 (d, J = 7.2 Hz, 2 H), 5.69 (d, J = 8.4 Hz, 1 H), 5.58 (t, J = 9.6 Hz, 1 H), 5.37 (d, J = 3.6 Hz, 1 H), 5.19 (s, 1 H), 5.11 (app t, J = 10.0 Hz, 1 H), 5.04–4.93 (m, 2 H), 4.64 (d, J = 12.0 Hz, 1 H), 4.36–4.18 (m, 4 H), 4.10–4.04 (m, 1 H), 3.77–3.59 (m, 4 H), 3.51 (td, J = 9.6, 4.8 Hz, 1 H), 2.13 (s, 3 H), 2.04 (s, 3 H), 1.84 (s, 3 H); 13C NMR (CDCl3, 100 MHz): δ = 170.7, 169.8, 169.7, 163.0 (d, JC-F = 248 Hz), 160.5, 153.7, 136.2, 135.7, 135.6, 133.1, 131.8, 130.8, 129.0, 128.2, 127.9, 125.5, 116.3, 116.1, 101.5, 99.0, 95.2, 87.0, 81.9, 77.2, 74.6, 72.3, 70.6, 70.0, 68.6, 68.4, 67.8, 62.1, 55.5, 20.8, 20.7, 20.3; IR (film, cm−1): ν = 3382, 2958, 1744, 1643, 1490, 1368, 1314, 1223; HRMS (ESI): calc. for C42H41Cl3F4N2O13S (M+H): 995.1409; found: 995.1434.
4-Fluorophenyl 3,4,6-Tri-O-Acetyl-2-Deoxy-2-o-Trifluoromethylbenzylideneamino-1-Thiol-α-d-Glucopyranoside 29
Viscous oil: 2 mg, 9%, α only; 1H NMR (CDCl3, 400 MHz):22 δ = 8.65 (d, J = 2.0 Hz, 1 H), 8.27 (d, J = 8.0 Hz, 1 H), 7.69 (d, J = 8.0 Hz, 1 H), 7.64–7.53 (m, 2 H), 7.50–7.45 (m, 2 H), 7.03–6.97 (m, 2 H), 5.64 (t, J = 10.0 Hz, 1 H), 5.60 (d, J = 5.6 Hz, 1 H), 5.14 (app t, J = 10.0 Hz, 1 H), 4.71–4.63 (m, 1 H), 4.30 (dd, J = 12.4, 5.2 Hz, 1 H), 4.03–3.94 (m, 2 H), 2.06 (s, 3 H), 2.02 (s, 3 H), 1.89 (s, 3 H); 13C NMR (CDCl3, 100 MHz): δ = 170.6, 169.9, 169.7, 161.2, 160.4, 134.5, 134.4, 133.1, 132.3, 131.0, 129.1, 129.0, 127.9, 125.5 (q, JC-F = 5.7 Hz), 116.3, 116.0, 86.8, 72.1, 71.6, 68.7, 68.3, 62.2, 20.72, 20.69, 20.4; IR (film, cm−1): ν = 2922, 1748, 1644, 1491, 1368, 1315, 1227; HRMS (ESI): calc. for C26H26F4NO7S (M+H): 572.1366; found: 572.1368.
2-Fluorophenyl 3,4,6-Tri-O-Acetyl-2-Deoxy-2-o-Trifluoromethylbenzylideneamino-α-d-Glucopyranosyl-(1→3)-4,6-O-Benzylidene-2-Deoxy-2-(2,2,2-Trichloroethyl)amino-1-Thiol-β-d-Glucopyranoside 23
Viscous oil: 30 mg, 60%, α only; [α]24D = 21.9 (c = 1.5, CH2Cl2); 1H NMR (CDCl3, 400 MHz): δ = 8.41 (d, J = 2.0 Hz, 1 H), 8.21 (d, J = 7.6 Hz, 1 H), 7.63–7.33 (m, 5 H), 7.20–7.03 (m, 5 H), 6.89 (d, J = 7.6 Hz, 2 H), 5.69 (d, J = 8.4 Hz, 1 H), 5.57 (t, J = 10.0 Hz, 1 H), 5.35 (d, J = 3.6 Hz, 1 H), 5.17 (s, 1 H), 5.14–4.97 (m, 3 H), 4.59 (d, J = 12.0 Hz, 1 H), 4.42–4.24 (m, 4 H), 4.14–4.05 (m, 1 H), 3.82–3.54 (m, 3 H), 3.63 (dd, J = 10.0, 3.6 Hz, 1 H), 3.52–3.44 (m, 1 H), 2.12 (s, 3 H), 2.03 (s, 3 H), 1.83 (s, 3 H); 13C NMR (CDCl3, 100 MHz): δ = 170.7, 169.8, 169.7, 162.0 (d, JC-F = 245 Hz), 160.5, 153.9, 136.2, 135.8, 131.9, 130.9, 130.8, 129.0, 128.35. 128.27, 127.9, 125.5, 124.8, 124.7, 116.2, 116.0, 101.6, 99.1, 95.1, 86.6, 82.0, 76.3, 74.7, 72.3, 70.7, 70.1, 68.6, 68.4, 67.9, 62.3, 55.9, 20.81, 20.76, 20.4; IR (film, cm−1): ν = 3332, 2965, 1743, 1642, 1474, 1368, 1314, 1224; HRMS (ESI): calc. for C42H42Cl3F4N2O13S (M+H): 995.1409; found: 995.1409.
2-Fluorophenyl 3,4,6-Tri-O-Acetyl-2-Deoxy-2-o-Trifluoromethylbenzylideneamino-1-Thiol-α-d-Glucopyranoside 30
Viscous oil: 1.3 mg, 4%, α only; 1H NMR (CDCl3, 400 MHz): δ = 8.67 (d, J = 2.0 Hz, 1 H), 8.29 (d, J = 8.0 Hz, 1 H), 7.74–7.53 (m, 5 H), 7.13–7.06 (m, 2 H), 5.76 (d, J = 6.0 Hz, 1 H), 5.70 (t, J = 9.6 Hz, 1 H), 5.18–5.02 (m, 2 H), 4.70–4.63 (m, 1 H), 4.27 (dd, J = 12.0, 4.8 Hz, 1 H), 4.03 (dd, J = 9.6, 5.6 Hz, 1 H), 3.85 (dd, J = 12.0, 2.0 Hz, 1 H), 2.06 (s, 3 H), 1.97 (s, 3 H), 1.90 (s, 3 H); IR (film, cm−1): ν = 2925, 1749, 1644, 1474, 1370, 1315, 1228; HRMS (ESI): calc. for C26H26F4NO7S (M+H): 572.1366; found: 572.1368.
2-Trifluoromethylphenyl 3,4,6-Tri-O-Acetyl-2-Deoxy-2-o-Trifluoromethylbenzylideneamino-α-d-Glucopyranosyl-(1→3)-4,6-O-Benzylidene-2-Deoxy-2-(2,2,2-Trichloroethyl)amino-1-Thiol-β-d-Glucopyranoside 24
Viscous oil: 31 mg, 61%, α only; [α]24D = 19.8 (c = 3.3, CH2Cl2); 1H NMR (CDCl3, 400 MHz): δ = 8.42 (d, J = 2.0 Hz, 1 H), 8.22 (d, J = 7.6 Hz, 1 H), 7.70 (d, J = 7.6 Hz, 1 H), 7.70 (d, J = 7.6 Hz, 1 H), 7.63 (d, J = 7.6 Hz, 1 H), 7.60–7.48 (m, 3 H), 7.46–7.40 (m, 1 H), 7.22–7.15 (m, 1 H), 7.10–7.03 (m, 2 H), 6.90 (d, J = 7.6 Hz, 2 H), 5.65–5.53 (m, 2 H), 5.34 (d, J = 4.0 Hz, 1 H), 5.20 (s, 1 H), 5.14–4.94 (m, 3 H), 4.53 (d, J = 12.0 Hz, 1 H), 4.44–4.37 (m, 1 H), 4.33–4.22 (m, 3 H), 4.13–4.05 (m, 2 H), 3.85 (t, J = 4.8 Hz, 2 H), 3.77 (t, J = 6.4 Hz, 1 H), 3.61 (dd, J = 10.0, 3.6 Hz, 1 H), 3.50 (td, J = 9.6, 4.8 Hz, 1 H), 2.11 (s, 3 H), 2.02 (s, 3 H), 1.82 (s, 3 H); 13C NMR (CDCl3, 100 MHz): δ = 170.7, 169.8, 169.6, 160.5, 154.0, 136.2, 135.5, 132.3, 131.9, 130.8, 128.9, 128.3, 128.0, 125.5, 101.6, 99.2, 95.0, 81.9, 77.2, 74.8, 72.3, 70.7, 70.0, 68.6, 68.4, 67.9, 62.5, 55.6, 20.77, 20.75, 20.4; IR (film, cm−1): ν = 3382, 2987, 1748, 1642, 1521, 1441, 1369, 1313, 1226; HRMS (ESI): calc. for C43H42Cl3F6N2O13S (M+H): 1045.1377; found: 1045.1379.
Phenyl 3,4,6-Tri-O-Benzyl-2-Deoxy-2-o-Trifluoromethylbenzylideneamino-α-D-Glucopyranosyl-(1→3)-4,6-O-Benzylidene-2-Deoxy-2-(2,2,2-Trichloroethyl)amino-1-Thiol-β-D-Glucopyranoside 39
Viscous oil: 36 mg, 64%, α:β = 6:1; 1H NMR (CDCl3, 400 MHz):23 δ = 8.53 (d, J = 2.0 Hz, 1 H), 8.33 (d, J = 7.6 Hz, 1 H), 7.62–7.13 (m, 26 H), 6.92 (d, J = 7.6 Hz, 2 H), 5.82 (d, J = 9.2 Hz, 1 H), 5.22 (d, J = 4.0 Hz, 1 H), 5.18 (s, 1 H), 4.87–4.04 (m, 13 H), 3.81–3.36 (m, 8 H); 13C NMR (CDCl3, 100 MHz): δ = 159.8, 154.1, 138.1, 138.0, 136.3, 133.6, 132.2, 132.9, 132.0, 131.8, 130.4, 128.9, 128.4, 128.3, 128.23, 128.18, 128.14, 128.10, 127.92, 127.91, 127.89, 127.8, 127.7, 127.5, 126.1, 125.5 (q, JC-F = 5.6 Hz), 125.4, 123.8 (d, JC-F = 273 Hz), 101.4, 99.5, 95.3, 87.8, 81.9, 80.9, 77.8, 77.2, 75.7, 75.6, 75.0, 74.6, 73.7, 70.9, 70.0, 68.5, 55.2; IR (film, cm−1): ν = 3389, 2867, 1743, 1640, 1454, 1368, 1314; HRMS (ESI): calc. for C57H55Cl3F3N2O10S (M+H): 1121.2595; found: 1121.2575.
Phenyl 3,4,6-Tri-O-Acetyl-2-Deoxy-2-o-Trifluoromethylbenzylideneamino-α-D-Glucopyranosyl-(1→3)-4-O-Benzyl-2-O-Benzoyl-6-O-tert-Butyldimethylsilyl-1-Thiol-β-D-Galactopyranoside 40
Viscous oil: 25 mg, 55%, α only; [α]24D = 73.9 (c = 1.8, CH2Cl2); 1H NMR (CDCl3, 400 MHz): δ = 8.62 (s, 1 H), 8.08 (d, J = 7.6 Hz, 2 H), 7.95 (d, J = 7.6 Hz, 1 H), 7.63–7.54 (m, 2 H), 7.50–7.15 (m, 14 H), 5.87–5.76 (m, 1 H), 5.49 (t, J = 9.6 Hz, 1 H), 5.18 (d, J = 11.2 Hz, 1 H), 5.12 (d, J = 3.2 Hz, 1 H), 4.99 (t, J = 9.6 Hz, 1 H), 4.82–4.75 (m, 1 H), 4.47 (d, J = 11.2, Hz, 1 H), 4.18–4.02 (m, 3 H), 3.94–3.55 (m, 6 H), 2.01 (s, 3 H), 1.79 (s, 3 H), 1.74 (s, 3 H), 0.85 (s, 9 H), 0.01 (s, 3 H), −0.01 (s, 3 H); 13C NMR (CDCl3, 100 MHz): δ = 170.5, 168.7, 168.3, 164.9, 161.3, 138.8, 133.1, 132.9, 132.1, 131.3, 130.7, 130.1, 129.8, 128.7, 128.6, 128.4, 128.0, 127.6, 127.5, 127.1, 125.4 (q, JC-F = 5.7 Hz), 98.2, 86.6, 81.0, 79.3, 77.2, 74.7, 72.8, 72.7, 70.5, 68.1, 67.9, 61.5, 61,4, 25.8, 20.6, 20.5, 20.3, 18.2, −5.4, −5.5; IR (film, cm−1): ν = 2928, 1728, 1668, 1602, 1365, 1314, 1263; HRMS (ESI): calc. for C52H61F3NO13SSi (M+H): 1024.3585; found: 1024.3585.
Phenyl 6-O-Acetyl-3,4-Di-O-Benzyl-2-Deoxy-2-o-Trifluoromethylbenzylideneamino-α-D-Glucopyranosyl-(1→3)-4-O-Benzyl-2-O-Benzoyl-6-O-tert-Butyldimethylsilyl-1-Thiol-β-D-Galactopyranoside 41
Viscous oil: 33 mg, 61%, α only; [α]24D = 30.4 (c = 0.1, CH2Cl2); 1H NMR (CDCl3, 400 MHz): δ = 8.69 (d, J = 2.0 Hz, 1 H), 8.02 (d, J = 6.8 Hz, 2 H), 7.95 (d, J = 7.6 Hz, 1 H), 7.60 (d, J = 7.6 Hz, 1 H), 7.48–7.32 (m, 7 H), 7.26–7.17 (m, 8 H), 7.14–7.07 (m, 6 H), 6.99–6.93 (m, 4 H), 5.92–5.76 (m, 1 H), 5.14 (d, J = 7.2 Hz, 1 H), 5.05 (d, J = 3.2 Hz, 1 H), 4.80 (d, J = 9.6 Hz, 1 H), 4.62 (d, J = 11.6 Hz, 1 H), 4.48 (d, J = 11.2 Hz, 1 H), 4.40 (d, J = 10.8 Hz, 1 H), 4.33 (d, J = 11.6 Hz, 1 H), 4.27 (d, J = 10.8 Hz, 1 H), 4.02–3.97 (m, 3 H), 3.92–3.88 (m, 1 H), 3,81 (dd, J = 12.0, 3.2 Hz, 1 H), 3.77–3.63 (m, 2 H), 3.49 (m, 3 H), 3.46 (t, J = 9.6 Hz, 1 H), 1.98 (s, 3 H), 0.83 (s, 9 H), 0.03 (s, 3 H), −0.03 (s, 3 H); 13C NMR (CDCl3, 100 MHz): δ = 170.5, 165.1, 160.5, 138.9, 138.1, 137.8, 133.1, 132.1, 132.0, 130.3, 130.0, 129.7, 128.7, 128.5, 128.3, 128.2, 128.1, 128.0, 127.9, 127.60, 127.57, 127.52, 127.47, 127.4, 127.1, 97.3, 86.8, 80.1, 79.2, 79.0, 77.2, 75.5, 75.0, 74.6, 74.3, 71.9, 69.6, 62.4, 61.3, 25.8, 20.8, 18.1, −5.4, −5.6; IR (film, cm−1): ν = 2925, 1735, 1642, 1454, 1362, 1314; HRMS (ESI): calc. for C62H69F3NO11SSi (M+H): 1120.4313; found: 1120.4309.
Phenyl 6-O-Acetyl-3,4-Di-O-Benzyl-2-Deoxy-2-o-Trifluoromethylbenzylideneamino-1-Thiol-α-D-Glucopyranoside 49
Viscous oil: 5 mg, 8%, α only; 1H NMR (CDCl3, 400 MHz): δ = 8.75 (d, J = 2.0 Hz, 1 H), 8.39 (d, J = 8.0 Hz, 1 H), 7.73–7.52 (m, 3 H), 7.48–7.44 (m, 2 H), 7.37–7.08 (m, 13 H), 5.55 (d, J = 5.2 Hz, 1 H), 4.88 (d, J = 10.8 Hz, 1 H), 4.69 (d, J = 10.4 Hz, 1 H), 4.63–4.52 (m, 3 H), 4.34–4.26 (m, 2 H), 4.17 (dd, J = 12.0, 2.0 Hz, 1 H), 3.95 (dd, J = 10.0, 5.6 Hz, 1 H), 3.65 (dd, J = 9.6, 8.8 Hz, 1 H), 3.35–3.26 (m, 1 H), 1.95 (s, 3 H); 13C NMR (CDCl3, 100 MHz): δ = 170.7, 159.5, 137.81, 137.75, 133.7, 132.2, 131.8, 130.7, 128.91, 128.85, 128.5, 128.3, 128.1, 128.0, 127.7, 127.1, 125.6 (q, JC-F = 5.7 Hz), 87.0, 81.8, 77.5, 75.23, 75.18, 75.1, 70.0, 63.1, 20.8; IR (film, cm−1): ν = 2923, 1742, 1641, 1454, 1364, 1314; HRMS (ESI): calc. for C36H35F3NO5S (M+H): 650.2188; found: 650.2195.
2-Trifluorophenyl 3,4,6-Tri-O-Acetyl-2-Deoxy-2-o-Trifluoromethylbenzylideneamino-α-D-Glucopyranosyl-(1→3)-4-O-Benzyl-2-O-Benzoyl-6-O-tert-Butyldimethylsilyl-1-Thiol-β-D-Galactopyranoside 42
Viscous oil: 38 mg, 71%, α only; [α]24D = 30.4 (c = 0.1, CH2Cl2); 1H NMR (CDCl3, 400 MHz): δ = 8.62 (d, J = 2.0 Hz, 1 H), 8.04 (d, J = 7.2 Hz, 2 H), 7.98 (d, J = 7.6 Hz, 2 H), 7.94 (d, J = 7.6 Hz, 1 H), 7.62–7.52 (m, 3 H), 7.47–7.38 (m, 3 H), 7.32–7.10 (m, 8 H), 5.99–5.87 (m, 1 H), 5.48 (t, J = 9.6 Hz, 1 H), 5.22 (d, J = 10.8 Hz, 1 H), 5.13 (d, J = 3.2 Hz, 1 H), 4.82–4.74 (m, 1 H), 4.79 (d, J = 8.8 Hz, 1 H), 4.15–4.02 (m, 3 H), 3.89–3.71 (m, 4 H), 3.65 (dd, J = 10.0, 3.2 Hz, 1 H), 3.58 (t, J = 6.4 Hz, 1 H), 2.01 (s, 3 H), 1.78 (s, 3 H), 1.73 (s, 3 H), 0.85 (s, 9 H), 0.02 (s, 3 H), −0.01 (s, 3 H); 13C NMR (CDCl3, 100 MHz): δ = 170.5, 169.7, 169.2, 164.8, 161.4, 138.7, 134.0, 133.0, 132.1, 132.0, 130.7, 129.9, 129.8, 128.5, 128.3, 128.1, 128.0, 127.3, 127.1, 126.4 (q, JC-F = 5.6 Hz), 125.4 (q, JC-F = 5.8 Hz), 98.1, 86.3, 80.9, 79.3, 77.2, 74.8, 72.6, 70.4, 68.1, 67.8, 61.5, 61,3, 25.8, 20.7, 20.5, 20.4, 18.2, −5.4, −5.5; IR (film, cm−1): ν = 2930, 1738, 1640, 1452, 1366, 1312, 1224; HRMS (ESI): calc. for C53H60F6NO13SSi (M+H): 1092.3459; found: 1092.3451.
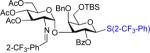
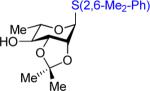
Phenyl 3,4,6-Tri-O-Acetyl-2-Deoxy-2-o-Trifluoromethylbenzylideneamino-α-D-Glucopyranosyl-(1→4)-2,3-Di-O-Isopropylidene-1-Thiol-α-L-Rhamnopyranoside 43
Viscous oil: 30 mg, 81%, α only; 1H NMR (CDCl3, 400 MHz): δ = 8.66 (d, J = 2.0 Hz, 1 H), 8.20 (d, J = 7.6 Hz, 1 H), 7.69 (d, J = 7.6 Hz, 1 H), 7.63–7.52 (m, 2 H), 7.48–7.44 (m, 2 H), 7.33–7.23 (m, 3 H), 5.76 (s, 1 H), 5.66 (t, J = 9.6 Hz, 1 H), 5.24 (app t, J = 9.6 Hz, 1 H), 5.02 (d, J = 3.6 Hz, 1 H), 4.53–4.44 (m, 2 H), 4.38–4.25 (m, 3 H), 4.15–4.06 (m, 2 H), 3.71 (dd, J = 10.0, 3.6 Hz, 1 H), 3.47 (dd, J = 10.0, 7.6 Hz, 1 H), 2.12 (s, 3 H), 2.04 (s, 3 H), 1.89 (s, 3 H), 1.55 (s, 3 H), 1.37 (s, 3 H), 1.17 (d, J = 6.0 Hz, 1 H); 13C NMR (CDCl3, 100 MHz): δ = 170.9, 170.0, 169.8, 161.3, 133.6, 132.2, 131.7, 131.4, 130.8, 130.0, 128.3, 127.4, 125.6 (q, JC-F = 5.4 Hz), 109.4, 100.0, 83.7, 82.1, 76.7, 76.5, 72.7, 70.8, 68.3, 67.7, 66.5, 61.5, 28.2, 26.5, 20.8, 20.7, 20.4, 16.9; IR (film, cm−1): ν = 2932, 1747, 1641, 1578, 1379, 1314, 1220; HRMS (ESI): calc. for C35H41F3NO11S (M+H): 740.2352; found: 740.2351.
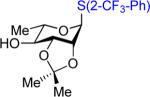
Phenyl 6-O-Acetyl-3,4-Di-O-Benzyl-2-Deoxy-2-o-Trifluoromethylbenzylideneamino-α-D-Glucopyranosyl-(1→4)-2,3-Di-O-Isopropylidene-1-Thiol-α-L-Rhamnopyranoside 44
Viscous oil: 30 mg, 72%, α only; 1H NMR (CDCl3, 400 MHz): δ = 8.78 (d, J = 2.0 Hz, 1 H), 8.36 (d, J = 7.6 Hz, 1 H), 7.30 (d, J = 7.6 Hz, 1 H), 7.67–7.53 (m, 2 H), 7.49–7.45 (m, 2 H), 7.38–7.13 (m, 13 H), 5.77 (s, 1 H), 4.94–4.87 (m, 2 H), 4.73 (d, J = 10.4 Hz, 1 H), 4.65–4.57 (m, 2 H), 4.51 (dd, J = 12.0, 2.0 Hz, 1 H), 4.40–4.25 (m, 6 H), 3.38 (dd, J = 10.0, 8.8 Hz, 1 H), 3.64 (dd, J = 10.0, 3.6 Hz, 1 H), 3.45 (dd, J = 10.0, 7.6 Hz, 1 H), 2.08 (s, 3 H), 1.56 (s, 3 H), 1.36 (s, 3 H), 1.13 (d, J = 6.4 Hz, 1 H); 13C NMR (CDCl3, 100 MHz): δ = 170.9, 160.6, 138.0, 137.9, 133.7, 133.5, 132.1, 131.4, 130.6, 129.0, 128.5, 128.29, 128.27, 128.2, 128.0, 127.9, 127.6, 127.4, 125.7 (q, JC-F = 5.6 Hz), 109.4, 100.8, 83.8, 81.8, 80.8, 76.5, 76.0, 75.1, 69.2, 66.7, 62.7, 28.2, 26.5, 21.0, 16.8; IR (film, cm−1): ν = 2926, 1739, 1638, 1455, 1381, 1314; HRMS (ESI): calc. for C45H49F3NO9S (M+H): 836.3080; found: 836.3088.
2-Trifluorophenyl 3,4,6-Tri-O-Acetyl-2-Deoxy-2-o-Trifluoromethylbenzylideneamino-α-D-Glucopyranosyl-(1→4)-2,3-Di-O-Isopropylidene-1-Thiol-α-L-Rhamnopyranoside 45
Viscous oil: 40 mg, 98%, α only; [α]24D = −48.9 (c = 0.8, CH2Cl2); 1H NMR (CDCl3, 400 MHz): δ = 8.66 (d, J = 2.0 Hz, 1 H), 8.21 (d, J = 7.6 Hz, 1 H), 7.75 (d, J = 7.6 Hz, 1 H), 7.68 (d, J = 7.6 Hz, 2 H), 7.61–7.46 (m, 3 H), 7.37–7.32 (m, 1 H), 5.79 (s, 1 H), 5.66 (t, J = 10.0 Hz, 1 H), 5.24 (t, J = 9.6 Hz, 1 H), 5.03 (d, J = 3.6 Hz, 1 H), 4.53–4.41 (m, 3 H), 4.24 (dd, J = 10.0, 6.0 Hz, 1 H), 4.28–4.20 (m, 1 H), 4.14 (dd, J = 10.0, 2.0 Hz, 1 H), 3.71 (dd, J = 10.0, 3.6 Hz, 1 H), 3.48 (dd, J = 10.0, 7.6 Hz, 1 H), 2.12 (s, 3 H), 2.04 (s, 3 H), 1.88 (s, 3 H), 1.55 (s, 3 H), 1.37 (s, 3 H), 1.17 (d, J = 6.4 Hz, 1 H); 13C NMR (CDCl3, 100 MHz): δ = 170.8, 170.0, 169.8, 161.1, 133.6, 133.3, 133.2, 132.2, 130.8, 129.4, 129.1, 128.4, 127.3, 126.8 (q, JC-F = 5.6 Hz), 125.6 (q, JC-F = 5.6 Hz), 125.4, 123.6 (d, JC-F = 275 Hz), 109.5, 100.0, 84.4, 81.9, 76.7, 76.5, 72.6, 70.9, 68.4, 67.8, 67.0, 61.6, 28.2, 26.6, 20.8, 20.7, 20.4, 16.9; IR (film, cm−1): ν = 2937, 1749, 1642, 1577, 1381, 1312, 1222; HRMS (ESI): calc. for C36H40F6NO11S (M+H): 808.2226; found: 808.2228.
2-Trifluorophenyl 6-O-Acetyl-3,4-Di-O-Benzyl-2-Deoxy-2-o-Trifluoromethylbenzylideneamino-α-D-Glucopyranosyl-(1→4)-2,3-Di-O-Isopropylidene-1-Thiol-α-L-Rhamnopyranoside 46
Viscous oil: 35 mg, 78%, α only; [α]24D = −7.6 (c = 1.0, CH2Cl2); 1H NMR (CDCl3, 400 MHz): δ = 8.76 (d, J = 2.0 Hz, 1 H), 8.35 (d, J = 7.6 Hz, 1 H), 7.76–7.44 (m, 6 H), 7.37–7.26 (m, 6 H), 7.21–7.12 (m, 5 H), 5.80 (s, 1 H), 4.93–4.87 (m, 2 H), 4.72 (d, J = 10.4 Hz, 1 H), 4.63–4.57 (m, 2 H), 4.61 (dd, J = 10.4, 6.0 Hz, 1 H), 4.43–4.18 (m, 6 H), 3.77 (t, J = 9.6 Hz, 1 H), 3.61 (dd, J = 10.0, 3.2 Hz, 1 H), 3.45 (dd, J = 10.0, 7.6 Hz, 1 H), 2.06 (s, 3 H), 1.55 (s, 3 H), 1.35 (s, 3 H), 1.12 (d, J = 6.4 Hz, 1 H); 13C NMR (CDCl3, 100 MHz): δ = 170.8, 160.4, 138.0, 137.9, 133.5, 133.3, 132.2, 132.1, 130.9, 130.6, 129.3, 128.5, 128.3, 128.2, 127.9, 127.8, 127.6, 127.1, 126.7 (q, JC-F = 5.8 Hz), 125.7 (q, JC-F = 5.5 Hz), 120.4, 109.5, 100.7, 84.5, 81.5, 80.8, 76.7, 76.5, 75.9, 75.1, 69.3, 67.2, 62.7, 28.1, 26.5, 20.9, 16.8; IR (film, cm−1): ν = 2935, 1740, 1641, 1454, 1381, 1312; HRMS (ESI): calc. for C46H48F6NO9S (M+H): 904.2954; found: 904.2957.
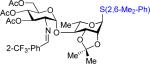
2,6-Dimethylphenyl 3,4,6-Tri-O-Acetyl-2-Deoxy-2-o-Trifluoromethylbenzylideneamino-α-d-Glucopyranosyl-(1→4)-2,3-Di-O-Isopropylidene-1-Thiol-α-l-Rhamnopyranoside 47
Viscous oil: 28 mg, 74%, α only; [α]24D = −70.8 (c = 4.8, CH2Cl2); 1H NMR (CDCl3, 400 MHz): δ = 8.66 (d, J = 2.0 Hz, 1 H), 8.21 (d, J = 8.0 Hz, 1 H), 7.69 (d, J = 7.6 Hz, 1 H), 7.63–7.53 (m, 2 H), 7.17–7.05 (m, 2 H), 5.67 (t, J = 10.0 Hz, 1 H), 5.40 (s, 1 H), 5.24 (app t, J = 9.6 Hz, 1 H), 5.02 (d, J = 3.6 Hz, 1 H), 4.55–4.42 (m, 3 H), 4.36 (dd, J = 7.2, 5.6 Hz, 1 H), 4.22–4.05 (m, 3 H), 3.72 (dd, J = 10.0, 3.6 Hz, 1 H), 3.43 (dd, J = 10.0, 7.6 Hz, 1 H), 2.53 (s, 6 H), 2.12 (s, 3 H), 2.05 (s, 3 H), 1.89 (s, 3 H), 1.53 (s, 3 H), 1.36 (s, 3 H), 1.15 (d, J = 6.4 Hz, 1 H); 13C NMR (CDCl3, 100 MHz): δ = 170.9, 170.0, 169.9, 161.2, 143.0, 133.2, 132.1, 131.5, 130.8, 128.8, 128.31, 128.28, 125.6 (q, JC-F = 5.6 Hz), 123.9 (d, JC-F = 263 Hz), 109.4, 99.9, 84.4, 81.9, 77.6, 76.5, 72.6, 70.8, 68.3, 67.72, 67.68, 61.6, 28.2, 26.6, 22.1, 20.8, 20.7, 20.4, 17.0; IR (film, cm−1): ν = 2936, 1746, 1641, 1459, 1378, 1314, 1220; HRMS (ESI): calc. for C37H45F3NO11S (M+H): 768.2665; found: 768.2668.
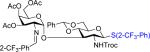
Phenyl 3,4,6-Tri-O-Acetyl-2-Deoxy-2-o-Trifluoromethylbenzylideneamino-α-d-Galactopyranosyl-(1→3)-4,6-O-Benzylidene-2-Deoxy-2-(2,2,2-Trichloroethyl)amino-1-Thiol-β-d-Glucopyranoside 54
Viscous oil: 65 mg, 67%, α only; [α]24D = 15.1 (c = 4.6, CH2Cl2); 1H NMR (CDCl3, 400 MHz): δ = 8.47 (d, J = 2.0 Hz, 1 H), 8.17 (d, J = 7.2 Hz, 1 H), 7.63 (d, J = 7.2 Hz, 1 H), 7.57–7.46 (m, 4 H), 7.39–7.30 (m, 3 H), 7.23–7.18 (m, 1 H), 7.14–7.08 (m, 2 H), 6.97 (d, J = 7.2 Hz, 2 H), 5.66 (d, J = 8.4 Hz, 1 H), 5.47–5.38 (m, 3 H), 5.19 (s, 1 H), 5.09–5.03 (m, 1 H), 5.00 (d, J = 12.0 Hz, 1 H), 4.63 (d, J = 12.0 Hz, 1 H), 4.54 (t, J = 6.4 Hz, 1 H), 4.31 (dd, J = 10.4, 4.8 Hz, 1 H), 4.38–4.24 (m, 1 H), 4.20–4.05 (m, 2 H), 3.85 (dd, J = 10.4, 3.6 Hz, 1 H), 3.77–3.68 (m, 2 H), 3.57–3.48 (m, 1 H), 2.15 (s, 3 H), 2.09 (s, 3 H), 1.83 (s, 3 H); 13C NMR (CDCl3, 100 MHz): δ = 170.5, 170.1, 169.7, 160.5, 153.9, 136.4, 132.4, 131.8, 130.6, 129.1, 128.9, 128.4, 128.2, 128.0, 125.5, 101.5, 99.7, 95.3, 87.5, 82.2, 76.1, 74.6, 70.0, 68.5, 68.2, 67.3, 66.8, 66.7, 62.3, 55.8, 20.9, 20.7, 20.3; IR (film, cm−1): ν = 3340, 2937, 1745, 1644, 1528, 1374, 1314, 1220; HRMS (ESI): calc. for C42H43Cl3F3N2O13S (M+H): 977.1504 ; found: 977.1509.
Phenyl 3,4,6-Tri-O-Acetyl-2-Deoxy-2-o-Trifluoromethylbenzylideneamino-1-Thiol-α-d-Galactopyranoside 60
Viscous oil: 10 mg, 16%, α only; 1H NMR (CDCl3, 400 MHz):22 δ = 8.69 (d, J = 2.0 Hz, 1 H), 8.30–8.26 (m, 1 H), 7.72–7.47 (m, 5 H), 7.33–7.22 (m, 3 H), 5.75 (d, J = 5.6 Hz, 1 H), 5.58–5.47 (m, 2 H), 4.84 (t, J = 6.4 Hz, 1 H), 4.19 (dd, J = 10.4, 5.6 Hz, 1 H), 4.13–4.04 (m, 2 H), 2.19 (s, 3 H), 1.92 (s, 3 H), 1.89 (s, 3 H); 13C NMR (CDCl3, 100 MHz): δ = 170.4, 170.1, 169.7, 160.3, 132.3, 131.9, 130.7, 129.2, 128.9, 127.2, 125.5, 86.8, 77.2, 69.6, 67.4, 66.9, 62.0, 20.7, 20.62, 20.55; IR (film, cm−1): ν = 2927, 1748, 1642, 1439, 1372, 1314, 1227; HRMS (ESI): calc. for C26H27 F3NO7S (M+H): 554.1460; found: 554.1460.
Phenyl 6-O-Acetyl-3,4-Di-O-Benzyl-2-Deoxy-2-o-Trifluoromethylbenzylideneamino-α-d-Galactopyranosyl-(1→3)-4,6-O-Benzylidene-2-Deoxy-2-(2,2,2-Trichloroethyl)amino-1-Thiol-β-d-Glucopyranoside 55
Viscous oil: 30 mg, 56%, α:β = 14:1; 1H NMR (CDCl3, 400 MHz):23 δ = 8.59 (d, J = 2.0 Hz, 1 H), 8.26 (d, J = 7.2 Hz, 1 H), 7.64–7.45 (m, 4 H), 7.36–7.17 (m, 14 H), 7.12–7.06 (m, 2 H), 6.95 (d, J = 7.6 Hz, 2 H), 5.60 (d, J = 8.8 Hz, 1 H), 5.36 (d, J = 3.6 Hz, 1 H), 5.20 (s, 1 H), 4.94 (t, J = 12.0 Hz, 2 H), 4.82 (d, J = 10.0 Hz, 1 H), 4.64–4.52 (m, 4 H), 4.33–4.25 (m,2 H), 4.20–3.96 (m, 6 H), 3.84–3.72 (m, 3 H), 3.53–3.46 (m, 1 H), 2.05 (s, 3 H); 13C NMR (CDCl3, 100 MHz): δ = 170.4, 160.4, 154.2, 138.2, 138.0, 136.3, 134.0, 133.1, 132.1, 131.7, 130.3, 129.0, 128.9, 128.5, 128.4, 128.3, 128.2, 128.0, 127.9, 127.5, 127.4, 125.6 (q, JC-F = 5.6 Hz), 125.5, 123.9 (d, JC-F = 273 Hz), 101.4, 100.1, 95.5, 81.9, 77.2, 76.1, 74.6, 73.2, 72.6, 70.3, 69.0, 68.5, 64.8, 55.5, 21.0; IR (film, cm−1): ν = 3350, 2924, 1741, 1640, 1524, 1370, 1313, 1234; HRMS (ESI): calc. for C52H51Cl3F3N2O11S (M+H): 1073.2231 ; found: 1073.2237.
Phenyl 6-O-Acetyl-3,4-Di-O-Benzyl-2-Deoxy-2-o-Trifluoromethylbenzylideneamino-1-Thiol-α-d-Galactopyranoside 61
Viscous oil: 2 mg, 6%, a only; 1H NMR (CDCl3, 400 MHz): δ = 8.54 (d, J = 2.0 Hz, 1 H), 8.20 (d, J = 7.6 Hz, 1 H), 7.71 (d, J = 7.6 Hz, 1 H), 7.63–7.45 (m, 4 H), 7.35–7.15 (m, 13 H), 4.95–4.99 (m, 2 H), 4.60 (d, J = 11.6 Hz, 1 H), 4.54 (d, J = 12.0 Hz, 1 H), 4.49 (d, J = 12.0 Hz, 1 H), 4.33 (dd, J = 11.2, 6.8 Hz, 1 H), 4.17 (dd, J = 11.2, 5.2 Hz, 1 H), 3.87–3.83 (m, 2 H), 3.77–3.72 (m, 2 H), 2.02 (s, 3 H); 13C NMR (CDCl3, 100 MHz): δ = 170.6, 160.6, 138.3, 137.7, 132.8, 132.2, 130.3, 129.7, 129.4, 129.1, 128.64, 128.59, 128.2, 128.0, 127.7, 127.6, 127.3, 125.6 (q, JC-F = 5.6 Hz), 124.0 (d, JC-F = 273 Hz), 86.3, 81.6, 76.4, 74.2, 72.4, 71.7, 70.4, 63.8, 20.8; IR (film, cm−1): ν = 2924, 1740, 1642, 1439, 1314, 1234; HRMS (ESI): calc. for C36H35F3NO5S (M+H): 650.2188 ; found: 650.2181.
2-Trifluoromethylphenyl 3,4,6-Tri-O-Acetyl-2-Deoxy-2-o-Trifluoromethylbenzylideneamino-α-d-Galactopyranosyl-(1→3)-4,6-O-Benzylidene-2-Deoxy-2-(2,2,2-Trichloroethyl)amino-1-Thiol-β-d-Glucopyranoside 56
Viscous oil: 38 mg, 73%, α only; [α]24D = 12.3 (c = 1.7, CH2Cl2); 1H NMR (CDCl3, 400 MHz): δ = 8.46 (d, J = 2.0 Hz, 1 H), 8.18 (d, J = 7.2 Hz, 1 H), 7.78 (d, J = 7.6 Hz, 1 H), 7.71 (d, J = 7.2 Hz, 1 H), 7.64 (d, J = 7.2 Hz, 1 H), 7.59–7.34 (m, 4 H), 7.23–7.18 (m, 1 H), 7.13–7.07 (m, 2 H), 6.94 (d, J = 7.2 Hz, 2 H), 5.64 (d, J = 8.8 Hz, 1 H), 5.48–5.36 (m, 3 H), 5.20 (s, 1 H), 5.08 (d, J = 12.0 Hz, 1 H), 4.92–4.83 (m, 1 H), 4.64–4.60 (m, 1 H), 4.56 (d, J = 12.0 Hz, 1 H), 4.28 (dd, J = 10.4, 4.8 Hz, 1 H), 4.21–4.03 (m, 3 H), 3.95–3.73 (m, 4 H), 3.52–3.44 (m, 1 H), 2.15 (s, 3 H), 2.12 (s, 3 H), 1.83 (s, 3 H); 13C NMR (CDCl3, 100 MHz): δ = 170.5, 170.1, 169.7, 160.6, 154.1, 136.3, 132.2, 131.8, 130.6, 129.0, 128.45, 128.36, 128.0, 126.9 (q, JC-F = 5.6 Hz), 125.5, 101.6, 99.8, 95.1, 82.0, 77.2, 76.1, 74.7, 70.0, 68.4, 68.2, 67.2, 67.0, 66.9, 60.4, 55.5, 21.0, 20.8, 20.4; IR (film, cm−1): ν = 3339, 2927, 1745, 1644, 1526, 1374, 1312, 1225; HRMS (ESI): calc. for C43H42Cl3F6N2O13S (M+H): 1045.1377; found: 1045.1367.
Phenyl 3,4,6-Tri-O-Acetyl-2-Deoxy-2-o-Trifluoromethylbenzylideneamino-α-d-Galactopyranosyl-(1→3)-4-O-Benzyl-2-O-Benzoyl-6-O-tert-Butyldimethylsilyl-1-Thiol-β-d-Galactopyranoside 57
Viscous oil: 46 mg, 52%, α:β = 8:1; 1H NMR (CDCl3, 400 MHz):23 δ = 8.66 (d, J = 2.0 Hz, 1 H), 8.12–8.07 (m, 2 H), 7.95 (d, J = 7.6 Hz,1 H), 7.66–7.18 (m, 18 H), 5.79 (t, J = 9.6 Hz, 1 H), 5.41 (dd, J = 3.2, 10.8 Hz, 1 H), 4.83–4.77 (m, 1 H), 4.44 (d, J = 11.6 Hz, 1 H), 4.20 (t, J = 8.0 Hz, 1 H), 4.11–4.03 (m, 2 H), 3.94–3.85 (m, 2 H), 3.80–3.67 (m, 3 H), 3.62–3.57 (m, 1 H), 2.08 (s, 3 H), 1.96 (s, 3 H), 1.82 (s, 3 H), 0.86 (s, 9 H), 0.02 (s, 3 H), −0.01 (s, 3 H); 13C NMR (CDCl3, 100 MHz): δ = 170.1, 169.9, 169.2, 165.0, 161.3, 138.8, 133.1, 132.8, 132.5, 132.2, 132.1, 131.9, 130.5, 130.0, 129.7, 128.7, 128.5, 128.0, 127.5, 127.1, 125.4 (q, JC-F = 5.7 Hz), 98.8, 86.7, 80.9, 79.3, 77.2, 74.7, 73.0, 68.1, 67.9, 67.0, 66.7, 61.5, 61.0, 25.8, 20.7, 20.6, 20.3, 18.2, −5.4, −5.5; IR (film, cm−1): ν = 2929, 1747, 1642, 1453, 1371, 1314, 1249; HRMS (ESI): calc. for C52H61F3NO13SSi (M+H): 1024.3585; found: 1024.3581.
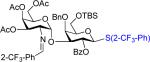
Phenyl 6-O-Acetyl-3,4-Di-O-Benzyl-2-Deoxy-2-o-Trifluoromethylbenzylideneamino-α-d-Galactopyranosyl-(1→3)-4-O-Benzyl-2-O-Benzoyl-6-O-tert-Butyldimethylsilyl-1-Thiol-β-d-Galactopyranoside 58
Viscous oil: 29 mg, 51%, α only; [α]24D = 116.3 (c = 0.3, CH2Cl2); 1H NMR (CDCl3, 400 MHz): δ = 8.73 (d, J = 2.0 Hz, 1 H), 8.03–7.94 (m, 3 H), 7.61–7.56 (m, 2 H), 7.46–7.05 (m, 24 H), 5.82 (t, J = 9.6 Hz, 1 H), 5.13–5.07 (m, 2 H), 4.83–4.74 (m, 2 H), 4.42–4.34 (m, 3 H), 4.26 (d, J = 11.2 Hz, 1 H), 4.10–3.93 (m, 8 H), 3.75–3.66 (m, 2 H), 3.57 (t, J = 10.8 Hz, 1 H), 1.91 (s, 3 H), 0.84 (s, 9 H), 0.00 (s, 3 H), −0.02 (s, 3 H); 13C NMR (CDCl3, 100 MHz): δ = 170.1, 164.9, 160.9, 138.9, 138.1, 133.5, 133.2, 132.1, 131.9, 130.1, 130.0, 129.7, 128.7, 128.4, 128.29, 128.27, 128.2, 127.9, 127.7, 127.5, 127.4, 125.3 (q, JC-F = 5.3 Hz), 97.6, 86.9, 79.3, 78.5, 77.2, 74.6, 74.4, 73.3, 72.7, 71.9, 70.4, 69.1, 63.0, 61.4, 25.8, 20.8, 18.2, −5.4, −5.6; IR (film, cm−1): ν = 2927, 1733, 1638, 1454, 1314, 1261; HRMS (ESI): calc. for C62H69F3NO11SSi (M+H): 1120.4313; found: 1120.4311.
2-Trifluoromethylphenyl 3,4,6-Tri-O-Acetyl-2-Deoxy-2-o-Trifluoromethylbenzylideneamino-α-d-Galactopyranosyl-(1→3)-4-O-Benzyl-2-O-Benzoyl-6-O-tert-Butyldimethylsilyl-1-Thiol-β-d-Galactopyranoside 59
Viscous oil: 34 mg, 62%, α only; [α]24D = 85.5 (c = 1.6, CH2Cl2); 1H NMR (CDCl3, 400 MHz): δ = 8.64 (d, J = 2.0 Hz, 1 H), 8.07–7.92 (m, 4 H), 7.63–7.54 (m, 3 H), 7.46–7.38 (m, 3 H), 7.32–7.15 (m, 8 H), 5.90 (t, J = 9.6 Hz, 1 H), 5.38 (dd, J = 3.2, 10.8 Hz, 1 H), 5.16–5.10 (m, 2 H), 5.03 (d, J = 2.0 Hz, 1 H), 4.78 (d, J = 10.0 Hz, 1 H), 4.44 (d, J = 10.8 Hz, 1 H), 4.18 (t, J = 7.2 Hz, 1 H), 4.10–4.02 (m, 2 H), 3.88–3.67 (m, 5 H), 3.58 (t, J = 6.4 Hz, 1 H), 2.07 (s, 3 H), 1.95 (s, 3 H), 1.80 (s, 3 H), 0.85 (s, 9 H), 0.02 (s, 3 H), −0.01 (s, 3 H); 13C NMR (CDCl3, 100 MHz): δ = 170.2, 170.0, 169.2, 165.0, 161.4, 138.6, 134.1, 133.1, 132.1, 132.0, 130.5, 130.3, 129.7, 129.6, 129.1, 128.7, 128.5, 128.3, 128.1, 127.8, 127.3, 127.1, 126.4 (q, JC-F = 5.8 Hz), 125.4 (q, JC-F = 5.8 Hz), 122.7, 122.3 (d, JC–F = 272 Hz), 98.7, 86.3, 80.7, 79.3, 77.2, 74.8, 72.7, 68.0, 67.8, 67.0, 66.6, 61.5, 61.1, 25.8, 20.68, 20.66, 20.3, 18.2, −5.4, −5.5; IR (film, cm−1): ν = 2929, 1746, 1641, 1452, 1372, 1312, 1249; HRMS (ESI): calc. for C53H60F6NO13SSi (M+H): 1092.3459; found: 1092.3456.
Phenyl 3,4,6-Tri-O-Acetyl-2-Deoxy-2-Azido-α-d-Glucopyranosyl-(1→3)-4,6-O-Benzylidene-2-Deoxy-2-(2,2,2-Trichloroethyl)amino-1-Thiol-β-d-Glucopyranoside 68
Viscous oil: 15 mg, 18%, α only; [α]24d = 60.8 (c = 0.5, CH2Cl2); 1H NMR (CDCl3, 400 MHz): δ = 7.52–7.45 (m, 4 H), 7.37–7.32 (m, 6 H), 5.65–5.57 (m, 2 H), 5.45–5.36 (m, 2 H), 5.13–5.01 (m, 2 H), 4.90 (d, J = 12.0 Hz, 1 H), 4.65 (d, J = 12.0 Hz, 1 H), 4.40 (dd, J = 10.4, 4.8 Hz, 1 H), 4.34–4.23 (m, 2 H), 4.16–4.11 (m, 1 H), 4.01 (dd, J = 12.0, 3.2 Hz, 1 H), 3.85–3.77 (m, 2 H), 3.60–3.47 (m, 2 H), 3.19 (dd, J = 10.8, 4.0 Hz, 1 H), 2.09 (s, 3 H), 2.07 (s, 3 H), 2.03 (s, 3 H); 13C NMR (CDCl3, 100 MHz): δ = 170.7, 170.0, 169.5, 153.7, 136.8, 132.5, 132.0, 129.2, 128.34, 128.32, 125.9, 101.4, 98.9, 95.1, 86.8, 74.6, 70.0, 69.7, 68.5, 68.4, 67.6, 62.0, 60.5, 55.9, 20.8, 20.7, 20.6; IR (film, cm−1): ν = 2923, 2108, 1748, 1453, 1367, 1215; HRMS (ESI): calc. for C34H37Cl3N4o13SK (M+K): 885.0781; found: 885.0792.
Phenyl 3,4,6-Tri-O-Acetyl-2-Deoxy-2-Azido-1-Thiol-α-d-Glucopyranoside 69
Viscous oil: 16 mg, 40%, α only; 1H NMR (CDCl3, 400 MHz): δ = 7.53–7.46 (m, 2 H), 7.38–7.29 (m, 3 H), 5.64 (d, J = 5.6 Hz, 1 H), 5.34 (dd, J = 10.4, 9.2 Hz, 1 H), 5.04 (dd, J = 10.4, 9.6 Hz, 1 H), 4.67–4.56 (m, 1 H), 4.30 (dd, J = 12.4, 4.8 Hz, 1 H), 4.09 (dd, J = 10.4, 5.6 Hz, 1 H), 4.03 (dd, J = 12.4, 2.0 Hz, 1 H), 2.11 (s, 3 H), 2.06 (s, 3 H), 2.04 (s, 3 H); 13C NMR (CDCl3, 100 MHz): δ = 170.5, 169.8, 134.1, 132.2, 129.2, 128.1, 86.4, 72.0, 68.6, 68.4, 61.9, 61.6, 20.7, 20.6; IR (film, cm−1): ν = 2918, 2109, 1748, 1367, 1227; HRMS (ESI): calc. for C18H21N3O7NaS (M+Na): 446.0998; found: 446.0997.
2-Trifluoromethylphenyl 3,4,6-Tri-O-Acetyl-2-Deoxy-2-Azido-α-d-Glucopyranosyl-(1→3)-4,6-O-Benzylidene-2-Deoxy-2-(2,2,2-Trichloroethyl)amino-1-Thiol-β-d-Glucopyranoside 70
Viscous oil: 6 mg, 13%, α only; [α]24D = 28.0 (c = 0.1, CH2Cl2); 1H NMR (CDCl3, 400 MHz): δ = 7.79 (d, J = 7.6 Hz, 1 H), 7.71 (d, J = 7.6 Hz, 1 H), 7.55–7.35 (m, 7 H), 5.65–5.60 (m, 2 H), 5.44–5.36 (m, 2 H), 5.01 (app t, J = 10.0 Hz, 1 H), 4.93 (d, J = 12.0 Hz, 1 H), 4.57 (d, J = 12.0 Hz, 1 H), 4.39 (dd, J = 10.8, 4.8 Hz, 1 H), 4.28–4.17 (m, 2 H), 4.07–4.03 (m, 1 H), 3.93–3.81 (m, 3 H), 3.70–3.52 (m, 3 H), 317 (dd, J = 10.8, 3.6 Hz, 1 H), 2.09 (s, 3 H), 2.07 (s, 3 H), 2.03 (s, 3 H); 13C NMR (CDCl3, 100 MHz): δ = 170.5, 170.0, 169.5, 154.0, 136.7, 132.3, 129.2, 128.5, 128.3, 125.9, 101.5, 99.0, 94.9, 81.3, 77.2, 74.8, 69.7, 68.4, 67.7, 62.2, 60.4, 20.74, 20.68, 20.65; IR (film, cm−1): ν = 3346, 2923, 2108, 1745, 1536, 1371, 1314, 1260; HRMS (ESI): calc. for C35H36Cl3F3N4O13NaS (M+Na): 937.0915; found: 937.0922.
Phenyl 3,4,6-Tri-O-Acetyl-2-Deoxy-2-Azido-α-d-Glucopyranosyl-(1→4)-2,3-Di-O-Isopropylidene-1-Thiol-α-l-Rhamnopyranoside 71
Viscous oil: 17 mg, 49%, α only; 1H NMR (CDCl3, 400 MHz): δ = 7.52–7.43 (m, 2 H), 7.36–7.27 (m, 3 H), 5.75 (s, 1 H), 5.50 (t, J = 9.6 Hz, 1 H), 5.15 (app t, J = 10.0 Hz, 1H), 5.08 (d, J = 4.0 Hz, 1 H), 4.47–4.31 (m, 3 H), 4.26–4.17 (m, 2 H), 4.05 (dd, J = 2.0, 10.0 Hz, 1 H), 3.52–3.38 (m, 2 H), 2.104 (s, 3 H), 2.096 (s, 3 H), 2.05 (s, 3 H), 1.53 (s, 3 H), 1.35 (s, 3 H), 1.28 (d, J = 6.0 Hz, 3 H); 13C NMR (CDCl3, 100 MHz): δ = 170.7, 170.0, 169.7, 133.1, 131.9, 129.1, 127.7, 109.5, 98.5, 83.5, 82.2, 76.6, 76.3, 70.6, 67.9, 67.5, 66.0, 61.3, 61.1, 28.2, 26.5, 20.8, 20.7, 20.6, 17.2; IR (film, cm−1): ν = 2982, 2108, 1749, 1380, 1221; HRMS (ESI): calc. for C27H35N3O11NaS (M+Na): 632.1890; found: 632.1888.
3,4,6-Tri-O-Acetyl-2-Deoxy-2-o-Trifluoromethylbenzylideneamino-α-d-Glucopyranosyl-(1→4)-2,3-Di-O-Isopropylidene-l-Rhamnopyranosyl-(1→6)-1,2,3,4-Bis-(Di-O-Isopropylidene)-α-d-Galactopyranoside 64
After the solution of disaccharide thioglycoside 45 (16 mg, 0.02 mmol), galactosyl acceptor 63 (10 mg, 0.04 mmol) and 4 Å MS (100 mg) in 2.0 mL of dry CH2Cl2 under argon was stirred at room temperature for 1 h and then cooled to −78 °C, NIS (14 mg, 0.06 mmol) was added. The reaction was allowed to warm to room temperature and stirred for 20 min and was then cooled to — 78 °C. AgOTf (3 mg, 0.01 mg) was added to the reaction, and the resulting mixture was warmed to 0 °C for 1 h and then slowly to room temperature overnight. TLC showed that the reaction was not complete, an additional amount of NIS (10 mg, 0.04 mmol) and AgOTf (3 mg, 0.01 mg) were added. The solution was stirred at 35°C for 3 h. The reaction mixture was filtered through a Celite pad, evaporated, and purified by flash chromatography on silica gel (hexane/ethyl acetate = 3/1 →2/1 → 3/2) to afford trisaccharide 64 (15 mg, 85%, α:β = 3:2). α,α-64: [α]24D = 29.8 (c = 0.5, CH2Cl2); 1H NMR (CDCl3, 400 MHz): δ = 8.65 (d, J = 2.0 Hz, 1 H), 8.18–8.15 (m, 1 H), 7.72–7.67 (m, 1 H), 7.58–7.51 (m, 2 H), 5.64 (t, J = 9.6 Hz, 1 H), 5.54 (d, J = 4.8 Hz, 1 H), 5.23 (app t, J = 9.6 Hz, 1 H), 5.02–4.98 (m, 2 H), 4.63 (dd, J = 8.0, 2.4 Hz, 1 H), 4.53–4.42 (m, 2 H), 4.32 (dd, J = 10.0, 2.8 Hz, 1 H), 4.26–4.20 (m, 3 H), 4.14–4.08 (m, 1 H), 3.87–3.80 (m, 2 H), 3.70 (dd, J = 10.0, 3.2 Hz, 1 H), 3.57 (dd, J = 10.0, 6.8 Hz, 1 H), 3.37 (dd, J = 10.0, 7.6 Hz, 1 H), 2.12 (s, 3 H), 2.03 (s, 3 H), 1.88 (s, 3 H), 1.57 (s, 3 H), 1.53 (s, 3 H), 1.41 (s, 3 H), 1.34 (s, 6 H), 1.32 (s, 3 H), 1.20 (d, J = 6.4 Hz, 3 H); 13C NMR (CDCl3, 100 MHz): δ = 170.9, 170.0, 169.9, 161.2, 133.3, 132.0, 130.8, 129.3, 128.2, 125.6 (q, JC–F = 5.6 Hz), 124.0 (d, JC–F = 275 Hz), 109.3, 109.1, 108.7, 99.9, 96.9, 96.2, 81.7, 77.2, 76.0, 75.6, 72.7, 71.1, 70.9, 70.6, 70.4, 68.3, 67.6, 66.5, 65.7, 64.9, 61.6, 28.2, 26.4, 26.2, 26.0, 24.9, 24.5, 20.8, 20.7, 20.5, 16.9; IR (film, cm−): ν = 2934, 1752, 1640, 1455, 1381, 1314, 1242; HRMS (ESI): calc. for C41H55F3NO17 (M+H): 890.3422; found: 890.3420. α,β-64: [α]24d = 25.5 (c = 0.2, CH2Cl2);1H NMR (CDCl3, 400 MHz): δ = 8.65 (d, J = 2.0 Hz, 1 H), 8.16 (d, J = 7.2 Hz, 1 H), 7.60 (d, J = 7.2 Hz, 1 H), 7.62–7.51 (m, 2 H), 5.63 (t, J = 10.0 Hz, 1 H), 5.49 (d, J = 4.8 Hz, 1 H), 5.22 (t, J = 9.6 Hz, 1 H), 5.02 (d, J = 3.6 Hz, 1 H), 4.82 (d, J = 2.0 Hz, 1 H), 4.61 (dd, J = 10.0, 2.0 Hz, 1 H), 4.49–4.43 (m, 2 H), 4.35–4.22 (m, 4 H), 4.12–3.98 (m, 3 H), 3.77 (dd, J = 9.6, 8.8 Hz, 1 H), 3.71 (dd, J = 12.0, 3.2 Hz, 1 H), 3.57–3.44 (m, 2 H), 2.12 (s, 3 H), 2.03 (s, 3 H), 1.88 (s, 3 H), 1.57 (s, 3 H), 1.51 (s, 3 H), 1.44 (s, 3 H), 1.38 (s, 3 H), 1.35 (s, 3 H), 1.31 (s, 3 H), 1.25 (d, J = 6.0 Hz, 3 H); 13C NMR (CDCl3, 100 MHz): δ = 170.9, 170.0, 161.3, 130.8, 128.3, 110.7, 109.1, 108.6, 99.9, 98.8, 96.2, 81.5, 77.2, 74.6, 72.5, 70.7, 70.6, 70.5, 68.4, 67.8, 67.7, 65.5, 61.6, 28.0, 26.5, 26.1, 26.0, 24.8, 24.5, 20.8, 20.7, 20.5, 17.6; IR (film, cm−1): ν = 2927, 1752, 1644, 1456, 1381, 1315, 1223; HRMS (ESI): calc. for C41H55F3NO17 (M+H): 890.3422; found: 890.3428.
3,4,6-Tri-O-Acetyl-2-Deoxy-2-o-Trifluoromethylbenzylideneamino-α-d-Glucopyranosyl-(1→3)-4,6-O-Benzylidene-2-Deoxy-2-(2,2,2-Trichloroethyl)amino-β-d-Glucopyranosyl-(1→6)-1,2,3,4-Bis-(Di-O-Isopropylidene)-α-d-Galactopyranoside 65
Viscous oil: 5 mg, 81%, β only; α,β-65: [a]24D = 5.9 (c = 1.6, CH2Cl2); 1H NMR (CDCl3, 400 MHz): δ = 8.44 (d, J = 2.0 Hz, 1 H), 8.24 (d, J = 7.2 Hz, 1 H), 7.64–7.52 (m, 3 H), 7.21–7.16 (m, 1 H), 7.09 (t, J = 7.6 Hz, 1 H), 6.93 (d, J = 7.6 Hz, 2 H), 5.64 (d, J = 4.8 Hz, 1 H), 5.60 (t, J = 9.6 Hz, 1 H), 5.53 (d, J = 4.8 Hz, 1 H), 5.36 (d, J = 4.0 Hz, 1 H), 5.18 (s, 1 H), 5.12 (d, J = 7.2 Hz, 1 H), 5.09 (d, J = 9.6 Hz, 1 H), 4.82 (d, J = 8.4 Hz, 1 H), 4.61 (dd, J = 8.0, 2.4 Hz, 1 H), 4.53–4.14 (m, 7 H), 4.07–3.95 (m, 2 H), 3.82–3.40 (m, 7 H), 2.14 (s, 3 H), 2.03 (s, 3 H), 1.84 (s, 3 H), 1.58 (s, 3 H), 1.46 (s, 3 H), 1.35 (s, 3 H), 1.34 (s, 3 H);13C NMR (CDCl3, 100 MHz): δ = 170.7, 169.9, 169.6, 160.3, 136.4, 133.2, 131.9, 130.7, 129.0, 128.9, 128.3, 127.9, 125.5, 109.4, 108.6, 102.1, 101.5, 99.1, 96.2, 95.4, 83.3, 77.2, 75.5, 74.6, 72.3, 71.1, 70.8, 70.6, 70.3, 68.9, 68.6, 67.8, 65.8, 62.3, 59.3, 56.5, 26.2, 26.0, 24.3, 22.7, 20.9, 20.8, 20.4; IR (film, cm−1): ν = 3468, 2926, 1747, 1634, 1586, 1455, 1375, 1314; HRMS (ESI): calc. for C48H57Cl3F3N2O19 (M+H): 1127.2573; found: 1127.2569.
3,4,6-Tri-O-Acetyl-2-Deoxy-2-N-Acetyl-α-d-Glucopyranosyl-(1→3)-4,6-Di-O-Acetyl-2 Deoxy-2-(2,2,2-Trichloroethyl)amino-β-d-Glucopyranosyl-(1→6)-1,2,3,4-Bis-(Di-O-Isopropylidene)-α-d-Galactopyranoside 66
A 25 mL RBF was charged with 65 (5 mg, 0.0044 mmol, 1.0 equiv.), 6N HCl (4.5 μL, 0.027 mmol, 6.0 equiv.) and acetone (1.0 mL). The solution was stirred under reflux for 5 minutes, concentrated in vacuo. The crude amine salt was used directly without further purification. To another A 25 mL RBF was charged with this crude amine intermediate, acetic anhydrate (0.5 mL), and pyridine (1.0 mL). After the reaction mixture had been stirring at room temperature overnight, it was azeotroped with toluene for three times. The resulting residue was purified by silica gel flash chromatography (hexane/ethyl acetate = 1/2 → 1/3) to afford trisaccharide 66 (5 mg, 99%) as a viscous oil. 66: [α]24D = 12.3 (c = 0.6, CH2Cl2); 1H NMR (CDCl3, 400 MHz): δ = 5.75 (d, J = 8.4 Hz, 1 H), 5.66 (d, J = 9.6 Hz, 1 H), 5.20–4.99 (m, 5 H), 4.66 (d, J = 8.4 Hz, 1 H), 4.60 (dd, J = 8.0, 2.4 Hz, 1 H), 4.51 (d, J = 12.0 Hz, 1 H), 4.39 (dt, J = 10.0, 4.0 Hz, 1 H), 4.32 (dd, J = 5.2, 2.4 Hz, 1 H), 4.23–4.16 (m, 4 H), 4.09–3.94 (m, 4 H), 3.76–3.52 (m, 3 H), 2.14 (s, 3 H), 2.11 (s, 3 H), 2.04 (s, 3 H), 2.014 (s, 3 H), 2.009 (s, 3 H), 1.97 (s, 3 H), 1.56 (s, 3 H), 1.44 (s, 3 H), 1.34 (s, 3 H), 1.33 (s, 3 H);13C NMR (CDCl3, 100 MHz): δ = 171.0, 170.8, 170.6, 109.4, 108.7, 98.4, 96.2, 95.3, 77.2, 74.7, 71.6, 71.2, 71.1, 70.9, 70.6, 70.2, 69.2, 68.1, 68.0, 67.7, 61.9, 56.4, 51.2, 26.2, 25.0, 24.3, 23.0, 22.7, 20.84, 20.82, 20.74, 20.69, 20.6; IR (film, cm−1): ν = 3347, 2924, 1747, 1669, 1539, 1455, 1374, 1231; HRMS (ESI): calc. for C39H55Cl3N2O22Na (M+Na): 1031.2210; found: 1031.2197.
ACKNOWLEDGMENT
We would like to thank the University of Iowa and National Institutes of Health (R01 GM098285) for their financial support.
Footnotes
Supporting Information. Detailed experimental procedures and characterization data, including 1H-NMR and 13C-NMR spectra for all new compounds, are provided. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
Full text links
Read article at publisher's site: https://doi.org/10.1021/jo301050q
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3436940?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1021/jo301050q
Article citations
Highly stereoselective α-glycosylation with GalN3 donors enabled collective synthesis of mucin-related tumor associated carbohydrate antigens.
Chem Sci, 15(17):6552-6561, 02 Apr 2024
Cited by: 2 articles | PMID: 38699257 | PMCID: PMC11062124
Highly Stereoselective Synthesis of 2-Azido-2-Deoxyglycosides via Gold-Catalyzed SN2 Glycosylation.
CCS Chem, 5(12):2799-2807, 04 Dec 2023
Cited by: 1 article | PMID: 38435838
Recent Advances in Chemical Synthesis of Amino Sugars.
Molecules, 28(12):4724, 12 Jun 2023
Cited by: 0 articles | PMID: 37375279 | PMCID: PMC10305009
Review Free full text in Europe PMC
Towards a Systematic Understanding of the Influence of Temperature on Glycosylation Reactions.
Angew Chem Int Ed Engl, 61(15):e202115433, 15 Feb 2022
Cited by: 8 articles | PMID: 35032966 | PMCID: PMC9306470
Modulating Heparanase Activity: Tuning Sulfation Pattern and Glycosidic Linkage of Oligosaccharides.
J Med Chem, 63(8):4227-4255, 07 Apr 2020
Cited by: 7 articles | PMID: 32216347 | PMCID: PMC7376576
Go to all (16) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Nickel-catalyzed stereoselective glycosylation with C(2)-N-substituted benzylidene D-glucosamine and galactosamine trichloroacetimidates for the formation of 1,2-cis-2-amino glycosides. Applications to the synthesis of heparin disaccharides, GPI anchor pseudodisaccharides, and α-GalNAc.
J Am Chem Soc, 132(40):14288-14302, 01 Oct 2010
Cited by: 39 articles | PMID: 20860359
Stereoselective 1,2-cis glycosylation of 2-O-allyl protected thioglycosides.
Chemistry, 8(11):2608-2621, 01 Jun 2002
Cited by: 6 articles | PMID: 12180341
Stereoselective α-glycosylation of C(6)-hydroxyl myo-inositols via nickel catalysis-application to the synthesis of GPI anchor pseudo-oligosaccharides.
Carbohydr Res, 381:146-152, 21 Sep 2013
Cited by: 7 articles | PMID: 24121123
Venturing beyond Donor-Controlled Glycosylation: New Perspectives toward Anomeric Selectivity.
Acc Chem Res, 51(3):628-639, 22 Feb 2018
Cited by: 26 articles | PMID: 29469568
Review