Abstract
Free full text

B cell responses to HIV-1 infection and vaccination: pathways to preventing infection
Abstract
The B cell arm of the immune response becomes activated soon after HIV-1 transmission, yet the initial antibody response does not control HIV-1 replication, and it takes months for neutralizing antibodies to develop against the autologous virus. Antibodies that can be broadly protective are made only in a minority of subjects and take years to develop—too late to affect the course of disease. New studies of the earliest stages of HIV-1 infection, new techniques to probe the human B cell repertoire, the modest degree of efficacy in a vaccine trial, and new studies of human monoclonal antibodies that represent the types of immune responses an HIV-1 vaccine should induce are collectively illuminating paths that a successful HIV-1 vaccine might take.
Acute HIV-1 infection and vaccine development
A correlate of protection of successful viral vaccines is the induction of anti-virus neutralizing antibodies. However, the antibodies that are routinely induced in HIV-1 infection and vaccination are not broadly reactive to circulating strains of HIV-1, and T cell responses, although responsible for controlling virus replication, have not been definitively demonstrated to prevent HIV-1 infection. The obstacles to the development of an HIV-1 vaccine continue to be the extraordinary diversity of HIV-1 quasispecies (created by its error-prone reverse transcriptase as well as selected by host immune responses) that require broadly reactive responses to conserved HIV-1 epitopes, the ability of HIV-1 to integrate into the host genome and persist as a latent reservoir (see Glossary), the combination of HIV-1 induced rapid impairment of antigen-specific responses coupled with induction of explosive polyclonal immune activation and the rapid selection of HIV-1 escape mutants by immune responses. Thus, understanding the ineffective initial B cell response in acute HIV-1 infection (AHI) as well as understanding the regulation of broadly neutralizing antibodies (bnAbs, see Glossary) and why they rarely occur are keys to successful HIV-1 vaccine development. This review summarizes recent progress and insights in these critical areas of study.
B cell responses in AHI
The first detectable antibody response to HIV-1 is seen ~ 8 days after the first appearance of plasma viremia (see Glossary) in the form of immunoglobulin (Ig)M and IgG antibodies in complex with virus1 (Figure 1). The initial B cell plasma response to HIV-1 appears ~ 13 days after plasma viremia, is targeted to envelope glycoprotein (gp)-41 and unlike the first T cell response that occurs around the same time, does not select for virus escape mutants1, 2. Rather it is not until the appearance of the autologous neutralizing antibody response (see Glossary) three months after transmission that antibodies arise that can select for escape mutants in the transmitted/founder virus3, 4. However, most estimates of when viral integration and establishment of the latent pool of infected CD4+ T cells occurs suggest the latent pool is established early, within the first two or three weeks of HIV-1 infection5. Thus, for the first weeks after infection the systemic antibody response is targeted at non-native, non-neutralizing HIV-1 envelope epitopes and is ineffective in controlling plasma viremia1. The initial mucosal IgG and IgA response to HIV-1 is similarly targeted to envelope (Env) gp416. In both plasma and genital secretions, whereas the anti-Env IgG response is detectable soon after plasma viremia and remains elevated well after the AHI phase, anti-Env gp41 IgA plasma and mucosal responses arise at the time of IgG response but decline steadily6. The reasons for the anti-Env IgA decline is not clear but may relate to early damage to mucosal B cell populations7, loss of CD4+ T cell help8, or regulatory T cell induction.
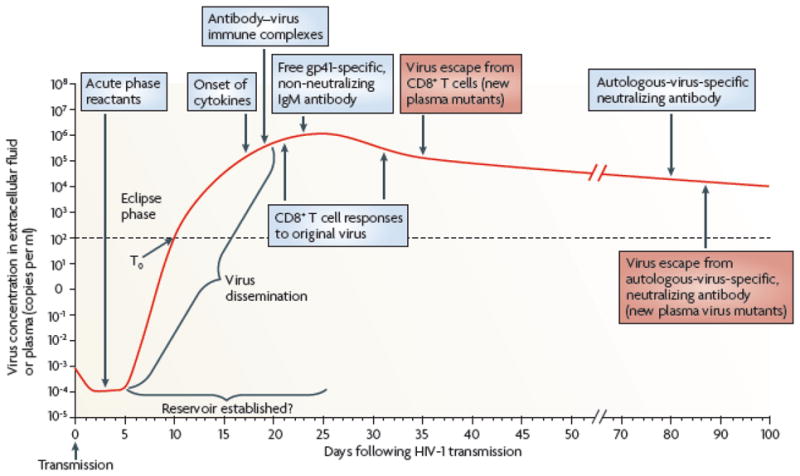
The first systemically detectable immune responses to HIV-1 infection are the increases in levels of acute-phase proteins in the plasma, which are observed when virus replication is still largely restricted to the mucosal tissues and draining lymph nodes (eclipse phase). When virus is first detected in the plasma (T0), broad and dynamic increases in plasma cytokine levels are also observed. Within days, as plasma viremia is still increasing exponentially, the first antibody–virus immune complexes are detected. Expansion of the earliest HIV-1-specific CD8+ T cell responses also commences prior to peak viremia, followed by detection of the first free gp41-specific but non-neutralizing IgM antibodies. Complete virus escape from the first CD8+ T cell responses can occur rapidly, within 10 days of T cell expansion. By this time, viral reservoirs exist, possibly becoming established within days of infection. The earliest autologous-virus-nAbs are detected around day 80 following infection, as viral loads are still declining prior to the onset of the viral set point. Antibody escape virus mutants emerge in the plasma within the following week. Figure is reproduced with permission from McMichael AJ, Haynes BF et al. Nature Medicine, 10:11–23, 2010. © Nature Publishing Group.
Similar to the timing of autologous neutralizing antibody responses (see Glossary), antibodies that can mediate antibody-dependent cellular cytotoxicity (ADCC, see Glossary) also arise during the first three months of acute infection. However, unlike the autologous neutralizing antibody response, the initial ADCC responses are cross reactive9, and therefore can target diverse circulating HIV-1 strains in addition to the autologous virus10. The elicitation of these cross-reactive ADCC antibodies appears too late to mediate early virus control but indicates that these responses are easier to elicit by the immune system than bnAbs, and thus, if present before transmission, could be a promising goal for vaccine-elicited antibody responses.
Simultaneous with the onset of acute plasma viremia and the first anti-HIV-1 responses, there is a striking burst in the production of plasma inflammatory cytokines11 (Figure 2) and soluble Tumor Necrosis Factor Apoptosis-Inducing Ligand (TRAIL) coupled with rises in plasma phosphatidylserine-expressing T cell apoptotic microparticles that are ~700X more prevalent in serum than virions and have the capability of being immunosuppressive11,12. A component of polyclonal B cell activation in acute HIV-1 infection could result in part from the early production of interferon-alpha (IFN-α)13 and interleukin (IL)-157,11. HIV-1 transmission can also cause generalized immune activation that can negatively impact antigen-specific immune responses and is strongly predictive of disease progression13–15.
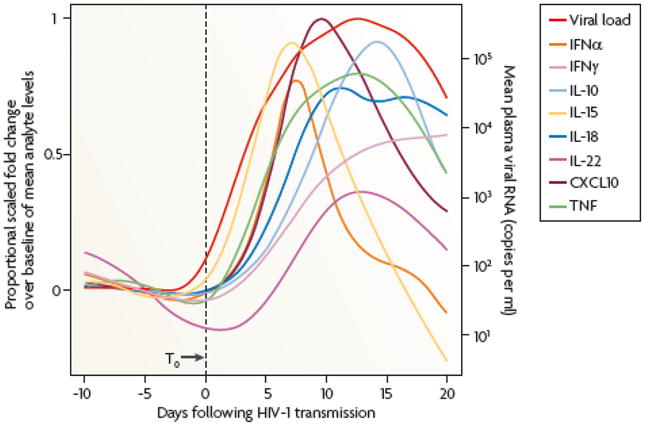
The relative kinetics of elevation of acute-phase proteins, cytokines and chemokines in the plasma during AHI. There are two initial waves of cytokines produces: IL-15and IFN-α, followed by TNF, IL-18 and IL-10. Abbreviations: CXCL10, CXC-chemokine ligand 10 The figure is reproduced with permission from Stacey AR, Borrow P et al. Journal of Virology, 83:3719-33, 2009. © American Society for Microbiology from reference 11.
Regarding the B cell responses to HIV-1 infection, a key question is: why is the initial antibody response ineffective in controlling HIV-1? Similarly, because virtually all infected subjects will eventually elicit autologous neutralizing antibody responses, why are these potentially protective antibody responses delayed? Although the answers to these questions are not fully understood, recent studies of the initial B cell function and mucosal microenvironments in AHI and early HIV-1 infection have been informative.
Regarding the ineffective nature of initial antibody responses to HIV-1, Liao et al have demonstrated that the initial non-neutralizing gp41 envelope response in AHI is derived from pre-existing gp41 cross-reactive memory B cells that acquire reactivity with autologous gp41 after initial triggering by nonHIV-1 antigens16. Bar et al. have shown that this initial gp41 response does not select for Env mutants. Rather it is not until the autologous antibody response arises ~3 months after transmission that an Env antibody response can select virus escape mutants2. The changes in B cell populations during AHI demonstrate a profound and immediate activating effect on host B cells. There are immediate decreases in percentages of naïve B cells in blood and reciprocal increases in blood and mucosal (terminal ileum) memory B cells/plasmablasts7. It has been suggested that there is a dearth of IgA antigen-specific responses in HIV-1 infection17. However, immediate IgA responses in plasma and mucosal secretions in AHI6 and in early infection have been found, including IgA antigen-specific plasma responses to Env gp41, Gag p55 and to Env gp120; in this study, the IgA and IgG responses to Env declined on anti-retroviral therapy, indicating loss of HIV-1 antigen drive6,7. Quantitative image analysis of terminal ileum lamina propria B cells 80 days after transmission showed no loss of IgM, IgG or IgA B cells compared to control tissues7. HIV-1 directly interacts with B cells and binds to B cell C-type lectins to induce polyclonal B cell activation and class switching18, 19. Thus, the initial B cell response to HIV-1 is ineffective in controlling HIV-1, due in part to gp41 activation of pre-existing HIV-1 Env cross-reactive B cell clones, and the inability of this initial antibody response to neutralize HIV-1. Epstein-Barr virus (EBV) preferentially transforms recently primed B cells. In early HIV-1 infection, EBV activation of terminal ileum B cells increased the numbers of IgG- and IgA-producing cultures compared to uninfected terminal ileum cultures, indicating that HIV-1 induced terminal ileum B cell class switching7. Moreover, 33% of the cultures were HIV-1 antigen specific, whereas 25% of the cultures were either cardiolipin, rheumatoid factor or influenza-reactive (compared to 2% in HIV-1 uninfected cultures), demonstrating a high degree of polyclonal B cell activation after HIV-1 infection7.
In peripheral lymph nodes, the predominant changes in AHI are in follicular dendritic cells (FDCs) and germinal centers (which become hyperplastic; see Glossary), and are followed in chronic infection by FDC destruction and germinal center involution20, 21. However, in Peyer’s patches and isolated lymphoid nodules of the terminal ileum in early HIV-1 infection, a loss of one-half the normal number of terminal ileum germinal centers was observed, with over 80% of germinal centers showing a degree of B cell apoptosis, FDC damage or both7 (Figure 3). Zhang et al.22 similarly observed simian immunodeficiency virus (SIV)-induced germinal center damage, loss if KI-67+ germinal center cells and damage to FDCs in acute infection in monkeys who were poor antibody responders and progressed to AIDS. Therefore, one reason for the delay in induction of autologous neutralizing antibodies may be early damage to generative B cell microenvironments in AHI. Thus, AHI is characterized by HIV-1-induced B cell antigen-specific and polyclonal B cell activation, an increase in the percent and absolute numbers of class-switched memory B cells and plasmablasts, considerable damage to terminal ileal germinal center FDCs as well as lamina propria, and germinal center B cell apoptosis. Interestingly, subjects with the greatest germinal center damage had higher IgG anti-Env antibody responses and higher plasma viral loads compared to _ENREF_7 those with minimal germinal center damage, suggesting that the degree of immune activation and antibody response resulted from the level of viremia and was associated with damage to B cell microenvironments (Figure 3).
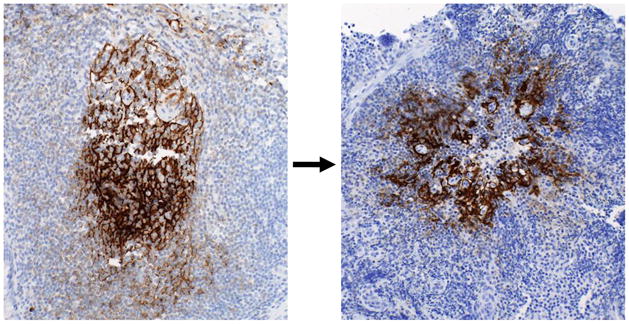
Terminal ileum tissues were stained with mAbs specific for follicular dendritic cells (FDCs). Left image shows a fine reticular pattern typical of a normal germinal center taken from terminal ileum Peyer’s patch of an uninfected control subject. Right image shows disruption of the normal germinal center architecture in tissue taken from a subject infected with HIV-1 approximately 105 days prior to biopsy. FDCs are clumped and have lost the typical reticular pattern of germinal center FDCs. Potential causes of germinal center damage include: direct virion binding to B cells and FDCs; inflammatory cytokines including INF-α, TNF, and IL-6; induction of TRAIL, Fas ligand, and TNF receptor-2; induction of CD8+ cytotoxic T lymphocytes; natural killer cell cytolysis; activation-associated exhaustion and apoptosis of B cells; B cell apoptosis due to loss of CD4 T cells; FDC loss due to B and CD4 T cell loss (i.e. loss of FDC positioning cues); and macrophage-mediated ADCC. Images taken at 20x; black bar equals 50 μm.
B cell response in chronic HIV infection
In untreated chronic HIV-1 infection, there is progressive destruction of B and T cell generative microenvironments in both peripheral and mucosal sites8, 20, 21. The changes in peripheral B cell pools include the loss of the antigen-specific memory B cells23, increase in the number of immature CD10+ transitional B cells, increase in exhausted tissue-like memory B cells, increase in circulating plasmablasts24 and an overall decrease in the resting memory B cell pool (reviewed in13). B cell exhaustion is coincident with the expression of FcRL4, an inhibitory Fc receptor-like molecule on tissue B cells25. The potential causes of HIV-1-induced B cell hyper-reactivity are listed in Box 1 along with the resulting effects on B cells13. In general, several factors likely act simultaneously that result in B cell dysfunction and dysregulation, often leading to hypergammaglobulinemia (see Glossary) and autoimmune phenomena—some of which are associated with autoimmune clinical syndromes26. For example, up to 40–50% of subjects with chronic HIV-1 infection make anti-cardiolipin and phosphatidylserine autoantibodies27. Recently, Moody and colleagues demonstrated that anti-phospholipid antibodies found in systemic lupus erythematosus (SLE; see Glossary) and anti-phospholipid syndrome patients can bind to lipids on monocytes via antibody Fab (Fragment, antigen binding) regions and to monocyte Fc receptors via antibody Fc (Fragment, crystallizable) regions, and induce high levels of monocyte-derived chemokines that potently and broadly inhibit HIV-1 infection in peripheral blood mononuclear cell (PBMC) assays28. Although the induction of anti-lipid antibodies is common29, it is unclear if the induction of the antibodies that bind lipids that also induce anti-HIV-1 chemokines is possible by a vaccine. Further, if possible to induce, it is not known whether these types of antibodies indeed protect against HIV-1 infection in vivo. Moreover, the neutralization properties of an antibody can be affected by the type of glycosylation of the Fc region of IgG30. It is interesting that patients with SLE and other autoimmune diseases have an unusually low incidence of HIV-1 infection31; we have proposed that these patients are able to make a more potent type of antibody such as polyreactive broad neutralizing antibodies that others cannot make32.
Broadly neutralizing antibody (bnAb) responses
There are multiple reasons why it is difficult to elicit bnAbs in the context of infection and HIV-1 Env vaccination (Box 2). Although most subjects infected with HIV-1 and experimentally vaccinated HIV-1 subjects do not make bnAbs, ~20% of chronically HIV-1 infected subjects will develop some degree of breadth of neutralizing antibodies ( i.e. antibodies that neutralize ~70–80% of HIV-1 strains with a degree of potency) and about 2% of chronically infected subjects have serum antibodies that broadly and potently neutralize most HIV-1 strains tested33–35.
An important development of the past year has been the application of recombinant human monoclonal antibody (mAb) isolation to HIV-1 vaccine development (reviewed in36). Several groups have either cultured single memory B cells or sorted Env-reactive memory B cells and have isolated new antibodies with significant neutralization breadth37–41. Box 3 summarizes the specificities of representative types of bnAbs and their targets.
HIV-1 bnAbs have unusual characteristics including i) high levels of somatic mutations ii) long heavy chain complementary determining region (HCDR)-3 (see Glossary), or iii) polyreactivity (see Glossary). All of the rare human bnAbs isolated thus far have at least one of these characteristics42, 43. The reason for the occurrence of rare B cell clones with unusually high levels of somatic mutations is unknown, but may reflect either a defect in the regulation of somatic mutation or the selection of B cells undergoing hypermutation. HIV-1 B cells from chronically infected patients might undergo multiple rounds of mutation and selection; however, human B cells expressing immunoglobulins with long HCDR3s (see Glossary) and/or with high affinity polyreactive properties are normally subjected to tolerance mechanisms (see Glossary)44–47. In experimental mouse models bearing transgenic autoantibodies, for example those bearing DNA autoantibodies (with long HCDR3s and high polyreactivity), B cells are either deleted in the bone marrow47, 48 or subjected to additional B cell tolerance mechanisms_ENREF_4749. However, not all polyreactive B cells undergo negative selection; indeed, in healthy people, up to 40% of B cells from the naïve, preselected repertoire are polyreactive, and a lower, but still significant subset of the postselection repertoire (i.e. 15%) retain polyreactivity46. It is important to note that deregulation of tolerized B cells that make high affinity polyreactive antibodies could lead to autoimmuity50 . Thus, polyreactivity represents a broad continuum of B cell specificities, of which only a subset trigger B cell tolerance mechanisms upon encounter with self-antigens during ontogeny.
Polyreactivity of HIV-1 antibodies can be functionally important because lipid polyreactivity of gp41 bnAbs is required for neutralization51. Because of the low number of Env trimer spikes on the HIV-1 virion, the likelihood of bivalent antibody/virion binding in the absence of antibody polyreactivity is small52. Nussenzweig and Mouquet have demonstrated that induction of polyreactive antibodies by HIV-1 facilitates Env antibody bivalent binding (avidity) on virions by allowing antibody cross-linking via Env and virion/host molecules53. One postulate of this is that the host needs to induce polyreactive antibodies to overcome HIV-1 escape from antibody avidity52. hat is to say, that because of few spikes on the surface of HIV-1 virions, it is difficult for a nonpolyreactive antibody to cross link Env spikes and increase binding by avidity. However, if an Env antibody is polyreactive, then one arm of an antibody can bind to nonEnv virion components while the other arm can bind to Env, thus binding more avidly to virions52–54.
We have previously postulated that tolerance mechanisms limit the expression of HIV-1 bnAbs with highly polyreactive characteristics32, 43. Direct experimental evidence for the role of tolerance in regulation of such bnAbs has now been obtained in mice whose B cells can only express the 2F5 variable region of the immunoglobulin heavy chain (VH)54,55. In this model, 2F5 VH-expressing B cells are largely regulated by clonal deletion in the bone marrow (i.e. central deletion), but importantly, a small yet significant fraction of B cells escape deletion, including a peripheral B cell population bearing anergic characteristics54,55. Thus, one area of intense research is the design of immunogens that can safely overcome peripheral anergy (see Glossary) of bnAb-expressing B cells.
The envelope-induced antibody response in chronic infection and experimental HIV-1 vaccination
Several anti-Env antibodies with distinct specificities are routinely induced in vaccination or infection, yet their role in protection against HIV-1 transmission remains unclear. With modest (31%) protection observed in the canary pox vector (ALVAC)/clades B/E gp120 RV144 Thai vaccine trial56 (see below), there is emerging interest in more readily induced antibodies as possible correlates of protection.
Autologous neutralizing antibodies
The initial antibody response capable of neutralizing the transmitted/founder virus (the autologous neutralizing antibody response) does not develop until approximately three months after transmission3,4. This autologous response is frequently targeted to variable Env regions but arises too late after transmission to prevent virus integration into the host genome and establishment of the latent pool of infected CD4+ T cells57. A critical question is whether Env targets of autologous neutralizing antibodies are sufficiently common to warrant design of a polyvalent immunogen to induce pre-existing antibodies that would neutralize a sufficient number of transmitted/founder virus strains to be clinically useful as a vaccine.
Antibodies that have potential to block HIV-1 transmission at mucosal surfaces
Hessell et al.58 have shown that Fc receptor binding of neutralizing antibodies is important for antibody protection against HIV-1 acquisition. A key question is whether non-neutralizing antibodies that can inhibit some of the early stages of HIV-1 transmission by founder viruses can have any effects in vivo in limiting acquisition of HIV. There are several steps where antibodies have the potential to interfere with the transmission event at mucosal sites42. Free virions or virions produced by infected cells can be aggregated by dimeric IgA or pentameric IgM and their movement through mucus inhibited59. IgG antibody from the plasma may move to the submucosal space and inhibit HIV interaction with dendritic cells and kill infected CD4 T cells (antibody dependent cellular cytoxicity or ADCC)60. IgG antibodies may also mediate phagocytosis of antibody-coated virions, mediate induction of anti-HIV-1 chemokines and other cytokines61, and mediate antibody dependent cell-mediated viral inhibition (ADCVI) 62,63. Of course, traditional neutralizing antibodies may inhibit infection of CD4 T cells at mucosal surfaces or in the submucosa64.
Informative to understanding the correlates of protection are studies of those HIV-1-infected subjects who spontaneously control HIV-1 plasma viremia (i.e. HIV-1 elite controllers), a subset of which have elevated levels of ADCC-mediating antibodies 65. In vaccine protection trials in nonhuman primates, ADCC and ADCVI activity of serum antibodies as well as epithelial transcytosis-blocking activity in mucosal secretions correlated with control of viral load66.
An important aspect of the anti-Env antibody response that occurs in the setting of chronic HIV-1 infection is that HIV-1 antibodies are short-lived and levels wax and wane—particularly in the presence of anti-retroviral treatment in which plasma viremia is lowered and therefore antigen drive is dimished67,68. Similarly, anti-Env antibody levels can be short-lived in the setting of vaccination as well67,69, although some studies have indicated that relatively durable responses (duration ~ 1 year) can be achieved with protein vaccination70,71. Further work is needed to determine whether like tetanus toxoid and other routine vaccines, immunization with optimal Env immunogens can induce long-lived plasma cells that can secrete antibody responses over years72.
The antibody response in the RV144 Thai trial
Preliminary analyses of the ALVAC/HIV prime, B/E envelope gp120 boost regimen in the RV144 trial demonstrate that binding antibodies to clade B and E gp120s were present in 99% of vaccinated subjects but titers waned 15-fold over 20 weeks (J. Kim and M. De Souza, personal communication). ADCC with gp120-coated targets were present in ~75% of vaccinees for clade B; as with binding antibodies, titers were not stable and waned over 20 weeks (M. De Souza and J. Kim, personal communication). nAbs targeted a subset of Tier 1 and 2 viruses (see Glossary) and were less potent than the failed Vax003 and Vax004 trials, which tested gp120 without the ALVAC/HIV prime 73 (reviewed in42). Thus, one hypothesis is that a correlate of protection in RV144 might be a short-lived antibody response that can neutralize or block an early phase of the HIV-1 mucosal transmission36
Concluding remarks
The B cell response to HIV-1 occurs within a few weeks after transmission but is non-neutralizing and does not initially select for escape mutants, unlike the initial T cell response. Soon after transmission, HIV-1 directly and indirectly induces polyclonal B cell activation. Although functional antibody responses that can mediate ADCC, neutralize macrophage infection and neutralize autologous HIV-1 appear within months of transmission, bnAbs, when made, appear much later and appear to have little impact on disease progression74–76. The destruction of the immune system starts early after transmission coincident with the appearance of the first anti-HIV-1 T and B cell responses. Thus, for a preventive vaccine to work, it must induce durable and long-lasting protective antibodies as well as memory CD4 and CD8 T cells at mucosal sites that must be present before the transmission event. The types of antibodies that are needed are preferably those that broadly neutralize HIV-1 strains across many clades. However, these antibodies are difficult to induce (Box 2). Thus, the way forward for utilizing what we have learned from the B cell response to HIV-1 infection is to determine how bnAbs develop in those rare subjects and to identify the stimuli that trigger B cell receptors on naïve B cells and allow them to evolve into memory B cells that make HIV bnAbs. That many of the broadly reactive neutralizing antibodies are polyreactive and are controlled by tolerance mechanisms, imply that it may be a delicate balance between breaking peripheral tolerance/anergy for safe induction of bnAbs and triggering of autoimmunity. In this regard, immunization regimens have been described that suggest use of potent cytokines and Toll-like receptor (TLR) agonists for induction of polyreactive antibodies77. Only by understanding the complex interrelationships of germinal center reactions, somatic hypermutation and human B cell tolerance mechanisms, including peripheral anergy, will we be able to generate the full spectrum of long-lasting protective antibodies at mucosal surfaces that are needed to prevent HIV-1 transmission.
Acknowledgments
The authors acknowledge George Shaw, Andrew McMichael, Myron Cohen, Joseph Sodroski, Norman Letvin, and David Goldstein for discussions, Kim McClammy for expert secretarial assistance, and apologize to our colleagues in the field of HIV vaccine development for not being able to reference all the appropriate work in this review due to space requirements. This work supported by a Collaboration for AIDS Vaccine Discovery grant from the Bill and Melinda Gates Foundation and the Center for HIV/AIDS Vaccine Immunology from the National Institutes of Health, National Institute of Allergy and Infectious Disease, Division of AIDS, (AI0678501).
Glossary
| Antibody dependent cellular cytotoxicity (ADCC) | a cytolytic immune response mediated by antibody binding to virus infected cells via the antibody Fab end, and binding to effector cells bearing Fc receptors for IgG that have the capacity to kill opsonized host target cells |
| Autologous neutralizing antibodies | antibodies produced by B cells that can neutralize an individual’s circulating transmitted/founder virus |
| B cell anergy | a type of tolerance mechanism in which recognition of self-antigens by B cells results in a state of functional inactivation, attenuation or modification of their subsequent responsiveness to antigenic stimuli. Anergic B cells are generally categorized by their low surface IgM levels, by their failure to secrete antibody, and by their refractory signaling properties |
| B cell tolerance | the failure by the immune system to produce functional B cells that recognize certain antigens. Antigens that most commonly induce tolerance are constituents of the body’s tissues (i.e. self-antigens), although foreign antigens can also induce tolerance under certain conditions. B cells subjected to tolerance are also referred to as being under “negative selection” because they are actively eliminated by various tolerance “mechanisms” such as clonal deletion, anergy or events that can modify immunoglobulin variable regions such as VH replacement or VL receptor editing |
| Broadly neutralizing antibodies (bnAbs) | antibodies produced by B cells that can neutralize diverse strains in multiple clades of HIV-1 outside of the virus strains infecting a subject |
| Germinal center | foci of B lymphocytes in immune tissues where B lymphocytes contact antigens, differentiate, and undergo antibody class-switching and somatic hypermutation |
| HCDR3 (heavy chain complementarity determining region) | a stretch of amino acids within the immunoglobulin heavy (H) chain Fab variable (V) third CDR that is involved in specific recognition of antigen |
| Hypergammaglobulianemia | increase in IgG antibody levels in plasma, usually associated with diseases with chronic inflammation such as autoimmune diseases and chronic infections |
| Latent reservoir | a CD4+ T cell with an integrated proviral copy of HIV-1 DNA capable of producing infectious virions upon stimulation. Latently infected CD4+ T cells are sheltered from both T and B cell responses and are resistant to anti-HIV-1 antiretroviral drugs. Polyreactivity, the ability of some antibodies to cross-react with multiple self and/or foreign antigens. Although a normal trait of many antibodies, high affinity polyreactivity can lead to clinically significant antibody reactivity with host molecules to cause clinical autoimmune disease syndromes. Of importance to HIV-1 B cell responses, HIV-1 selectively induces polyreactive antibodies, and many of the rare bnAbs, when induced, show varying degrees of polyreactivity. Some of these polyreactive antibodies, such as clinically relevant autoantibodies, are controlled by tolerance mechanisms |
| RV144 Thai HIV-1 vaccine efficacy trial | a pivotal HIV-1 vaccine clinical trial (nonreplicating avian pox clade E gp120 vector prime, HIV-1 Env clades B/E gp120 protein boost) performed by the U.S. Army in collaboration with the Thailand Ministry of Health in 16,000 subjects who, for the first time, showed some level of protection by any HIV-1 vaccine. However, the lower rate of infection in the vaccine recipients was modest, 31%, and the immune correlates of protection in this trial remain unknown |
| Systemic lupus erythematosus (SLE) | an autoimmune disease affecting many different organs that is associated with defects in maintenance of immune tolerance to self-antigens. Thus, in SLE, the immune system recognizes self-antigens as foreign leading to organ damage |
| Tier 1 HIV-1 viruses | HIV-1 strains that when grown in vitro as envelope pseudoviruses or as whole replicating viruses, are relatively easy to neutralize by infected patient sera antibodies or by envelope-induced antibodies |
| Tier 2 HIV-1 viruses | HIV-1 strain that when grown in vitro as envelope pseudoviruses or as whole replicating viruses, are relatively difficult to neutralize by infected patient sera antibodies or by envelope-induced antibodies |
| Viremia | the presence of HIV-1 virion in plasma as measured by assays to detect either viral proteins or RNA |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.molmed.2010.10.008
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3053087?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Article citations
Humanized Immunoglobulin Mice: Models for HIV Vaccine Testing and Studying the Broadly Neutralizing Antibody Problem.
Adv Immunol, 134:235-352, 01 Jan 2017
Cited by: 12 articles | PMID: 28413022 | PMCID: PMC5914178
Review Free full text in Europe PMC
Antiretroviral naive and treated patients: Discrepancies of B cell subsets during the natural course of human immunodeficiency virus type 1 infection.
World J Virol, 5(4):155-160, 01 Nov 2016
Cited by: 0 articles | PMID: 27878102 | PMCID: PMC5105048
Enhancing the Quality of Antibodies to HIV-1 Envelope by GagPol-Specific Th Cells.
J Immunol, 195(10):4861-4872, 14 Oct 2015
Cited by: 30 articles | PMID: 26466954
Competitive exclusion by autologous antibodies can prevent broad HIV-1 antibodies from arising.
Proc Natl Acad Sci U S A, 112(37):11654-11659, 31 Aug 2015
Cited by: 33 articles | PMID: 26324897 | PMCID: PMC4577154
Polyreactivity and autoreactivity among HIV-1 antibodies.
J Virol, 89(1):784-798, 29 Oct 2014
Cited by: 128 articles | PMID: 25355869 | PMCID: PMC4301171
Go to all (32) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Protein structures in PDBe
-
(1 citation)
PDBe - 2G12View structure
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Coadministration of CH31 Broadly Neutralizing Antibody Does Not Affect Development of Vaccine-Induced Anti-HIV-1 Envelope Antibody Responses in Infant Rhesus Macaques.
J Virol, 93(5):e01783-18, 19 Feb 2019
Cited by: 8 articles | PMID: 30541851 | PMCID: PMC6384077
Modulation of Vaccine-Induced CD4 T Cell Functional Profiles by Changes in Components of HIV Vaccine Regimens in Humans.
J Virol, 92(23):e01143-18, 12 Nov 2018
Cited by: 6 articles | PMID: 30209165 | PMCID: PMC6232489
Plasma IgG to linear epitopes in the V2 and V3 regions of HIV-1 gp120 correlate with a reduced risk of infection in the RV144 vaccine efficacy trial.
PLoS One, 8(9):e75665, 26 Sep 2013
Cited by: 184 articles | PMID: 24086607 | PMCID: PMC3784573
Progress in HIV vaccine development.
Hum Vaccin Immunother, 13(5):1018-1030, 10 Mar 2017
Cited by: 46 articles | PMID: 28281871 | PMCID: PMC5443372
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NIAID NIH HHS (4)
Grant ID: R01 AI087202
Grant ID: AI0678501
Grant ID: U19 AI067854-06
Grant ID: U19 AI067854




